Editor's note: Corrosion is one of the three main failure modes of metals. Stainless steel is often used to suppress corrosion of metals in more demanding environments. However, engineers have found that even with stainless steel, components can still corrode under certain conditions. When pitting corrosion occurs in stainless steel, many engineers are at a loss. The author believes that many engineers have misunderstandings when choosing stainless steel materials. This misunderstanding is that stainless steel is resistant to corrosion or even corrosion. I remember a saying like this: The man has tears and does not flick, just because he is not sad. This sentence is not used in stainless steel corrosion, stainless steel is not non-corrosive, just because it does not encounter a more harsh corrosive environment. Here we will focus on the local corrosion of stainless steel, hoping to relieve some of the doubts in this field.

For stainless steel materials containing chromium nickel, there are two main forms of corrosion: one is uniform corrosion and the other is localized corrosion. Rust in the ocean atmosphere is a typical example of general or uniform corrosion. Here the metal is uniformly etched over its entire surface. In this case, the steel surface forms a loose layer which is easily removed. Uniform corrosion is one of the easiest forms of corrosion because engineers can quantitatively determine the corrosion rate of metals and accurately predict the useful life of metals. Therefore, uniform corrosion is a form of corrosion that suffers from minimal ailments. Although it causes corrosion damage, it can be predicted and controlled.
However, the occurrence of localized corrosion often makes many engineers unprepared. This is because the damage caused by local corrosion is difficult to predict and the life of the equipment cannot be accurately calculated. One of the most annoying pittings is the most difficult one to deal with in local corrosion. Because of the thousand miles of the embankment, it collapsed in the ant hole. This so-called pitting is the ant hole on the embankment of a thousand miles.
In the process of corrosion of the metal, two reactions occur simultaneously on the electrode, one is a cathode reaction, the non-metal is reduced on the cathode, the non-metal is electron-derived, and the valence is lowered. The other is an anodic reaction. When an anodic reaction occurs, the metal loses electrons, the valence increases, and the metal ions are detached from the metal surface. What the author wants to say is that the corrosion of the metal depends on the reaction with the greatest corrosion resistance. Therefore, this also provides a main guiding ideology for solving metal corrosion problems.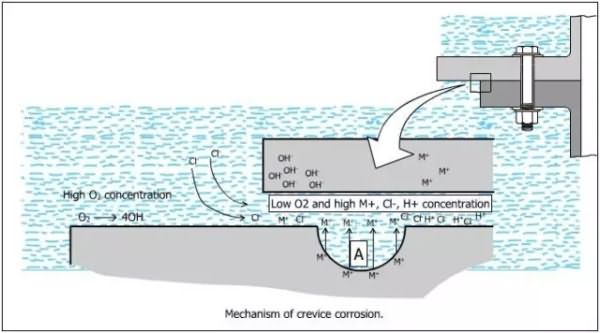
Corrosion resistant design using cathode and anode relationships. If a large cathode face is connected to a small anode face, a large current flow occurs between the anode and the cathode. This situation must be avoided. On the other hand, when we reverse the situation, that is, when a large anode surface is connected to a small cathode surface, a small current flow occurs between the two metals. This situation is what we expect. We design the welded metal joints in a certain container or tank as the cathode. The fastener device is designed such that the cathode fastener (small area) is joined to the anode member (large area). An example of this concept is the riveting of steel plates with copper rivets and exposure to seawater with low flow rates. The copper fixture is a small cathode face and the steel plate is a large anode face. This design is very convenient and produces good compatibility.
Pitting problem. Pitting can also occur in the absence of a gap on the metal surface. The occurrence of pitting may come from two factors: the chloride ion in the environment and the inhomogeneity of the microstructure or composition. The concentration of special corrosive agents such as chlorides will cause pitting of stainless steel. If the microstructure of the stainless steel is not uniform due to sensitization or the chromium or nickel content is not uniform, or even the ability to resist pitting is not achieved, pitting will occur. Defects on the metal surface can also cause pitting. For example, a defect in a protective oxide layer of stainless steel or nickel alloy. Pitting corrosion can be prevented by using an alloy that is highly resistant to corrosion or by eliminating chemical elements that cause pitting. Another aspect of controlling metal pitting is the elimination of cathode reactants in the ambient medium, which typically results in better oxygen removal. As the bottom of the pit tends to be anodized, the surrounding area of ​​the pit or slit tends to be cathodized, so that the relationship of the battery current is formed. When the corrosion in the pit or gap is further expanded, it becomes an autocatalytic reaction. Ferric ions interact with chloride ions to form ferric chloride. This reaction is repeated and rapidly produces metal perforation. Pitting or crevice corrosion is a very dangerous form of corrosion because it is highly localized and can quickly cause metal penetration damage.
Corrosion problems under scale. Just below the sediment or in the gap, the oxygen content in the solution is low, and the oxygen content in the large amount of solution outside the gap is high, which establishes a battery whose anode is under the sediment or in the gap and outside. It is the cathode. Inside the gap containing the chloride medium, the pH drops and the chloride concentrates. This acidic chloride condition results in accelerated corrosion and is automatically mediated. Then severe local corrosion occurred. An example of this form of corrosion is produced when a stainless steel fastener is placed on a stainless steel plate and exposed to chloride-containing water. Crevice corrosion can occur when the bolt head or gasket acts as the anode region. Preventing the formation of deposits and scales or using high alloy content materials will help reduce crevice corrosion.
Peel off corrosion. In this case, a loose, sheet-like etching layer is formed on the metal surface. Even at low speeds, the loose layer of corrosives is easily removed. The new, unetched metal is then exposed again, which will form a number of additional sheetlike layers. Again, these sheet layers are easily removed and the process continues. Exfoliation corrosion can be avoided by using an alloy that is not susceptible to chemical reactions.
Intergranular corrosion. Occurred in some special alloys, intergranular corrosion may occur when they are heated to their sensitive temperature zone during soldering or heat treatment. When, for example, certain stainless steel alloys are heated to 425-870 ° C, chromium carbides precipitate at grain boundaries. It leads to the occurrence of chromium-depleted regions near the carbides and affects the passivation of the grain boundary regions. In special media, such as nitric acid or high temperature water, corrosion in the low chromium zone may occur. The grains appear as a sugar-like surface which is easily rubbed off when rubbed with a sampler. Intergranular corrosion of stainless steel and nickel alloys can be avoided by using low carbon alloys, adding carbide forming elements such as titanium or tantalum, or by stabilizing annealing.
Stress corrosion cracking. A typical example is an adiabatic steam line made of AISI Type 316 stainless steel (UNS S31600). Chloride that may be present in the insulating material can be transferred to the metal surface when it is exposed to rain. This condition satisfies the conditions for stress corrosion cracking: a sensitive alloy - Type 316 stainless steel; a special etchant - chloride-containing water; and stress - cold worked or welded pipe. If a cross-section metallographic examination is performed through the crack zone, typical transgranularity (crossing the grain and grain boundaries) and branching cracks will be observed. This is the typical chloride stress corrosion crack of austenitic stainless steel. Eliminating any of the above three conditions can prevent the occurrence of stress corrosion cracking.
Oxygen content affects corrosion. Often, the fresh, clean water flowing into the power plant is not very corrosive. Steel works well in neutral water and its corrosion rate is directly related to the dissolved oxygen capacity. That is, the more the oxygen content, the higher the corrosion rate. Corrosion of steel is also related to pH. When the pH is high, the corrosion rate of steel is low. When the pH drops below 4, the steel will produce rapid corrosion.
Temperature also accelerates corrosion of the steel. When the temperature is raised from 72 °F to 104 °F (22 ~ 41 °C), it has a direct impact on the corrosion rate of steel. The flow rate has an opposite effect on the corrosion of the steel. When the flow rate of seawater is higher than about 3 feet per second (0.9 m/s), the corrosion of the steel is greatly accelerated. Mechanical removal of an unprotected corrosive will result in a high corrosion rate because the removal of corrosives exposes new metals with high corrosion rates. At the same time, a high flow rate will bring a lot of oxygen to the exposed surface of the metal. Therefore, more oxygen promotes an increase in the corrosion rate.
If the austenitic stainless steel breaks due to stress corrosion cracking, the alternative material to consider is duplex stainless steel. Due to their different composition and composition, they have higher mechanical properties at room temperature up to 600 °F (315 °C) compared to 316 stainless steel. They also have higher resistance to stress corrosion cracking. Dual phase alloys achieve higher resistance to pitting and crevice corrosion by increasing the chromium and molybdenum content.
The effect of chloride concentration on corrosion of stainless steel. When using 304 or 304L stainless steel in fresh water, the chloride content should be less than 200 ppm. After the components are manufactured, the residual iron must be removed. Because residual iron will act like a gap, it will also accelerate localized corrosion by reacting with chloride to form ferric chloride. The 304 pipe is periodically cleaned to remove deposits or deposits that can form gaps. Exposure of factory equipment manufactured by 304 or 304L to non-flowing water (eg, flow rates less than 0.9 m/s) should be avoided as this will deposit deposits on the metal surface. Microbial corrosion must also be controlled.
In order to successfully use 316L stainless steel in slightly salty water, the chloride content should be less than 1000 ppm unless the water has been completely deoxygenated. Deoxidized water will prevent pitting, cracking and stress corrosion of 316L stainless steel. In the production process of the factory equipment, the weld should be completely penetrated and smooth, in order to obtain the best anti-corrosion effect. Electrodes containing higher molybdenum or matching the weld should be used. It is important to clean the surface of the 316L stainless steel like the Type 304 and remove any residual iron. In general, the best way to remove residual iron is to use a HNO3-HF cleaner. In addition, any deposits should be removed regularly. It is important to take care to avoid water that is not flowing. The water flow rate should be at least 0.9 m/s during the shutdown of the equipment to prevent the formation of deposits.
Metal corrosion is often a very complex problem, and even some new forms of corrosion are not well recognized by the public. It is recommended that field engineers learn more about corrosion and protection to learn how to deal with metal components after they have corroded. I hope this article is helpful to everyone.
Beveling Machine,Plate Beveling Machine,Steel Plate Bevelling Machine,Pipe Beveller
Shandong EN FIN CNC Machinery Co., Ltd , https://www.sdfincncmachine.com