According to foreign media reports, lithium ions in next-generation batteries may be replaced by more abundant and environmentally friendly alkali metals or multivalent ions. However, the main challenge is to develop stable electrodes that can combine high energy density with fast charge and discharge rates. Recently, scientists in China and the United States have developed a high-performance electrode made of organic polymers, which can be used in low-cost, environmentally friendly and durable sodium ion batteries.
(Source: Wiley)
At present, lithium-ion batteries are the most advanced technology that can be used in portable devices, energy storage systems and electric vehicles, and lithium-ion battery technology won the Nobel Prize this year. However, next-generation batteries are expected to use cheaper, safer, and more environmentally friendly materials to achieve higher energy density and capacity. At present, the most developed battery types basically use the same charge and discharge technology as lithium batteries, but usually lithium ions are replaced by cheap metal ions such as sodium, magnesium and aluminum. However, this substitution also requires major adjustments to the electrode materials.
Organic compounds are good electrode materials. First, they do not contain harmful and expensive heavy metals; second, they can be used for different purposes. However, the disadvantage is that it will dissolve in the liquid electrolyte, resulting in electrode instability.
Chunsheng Wang and his team at the University of Maryland in the United States have collaborated with an international scientific team to introduce an organic polymer that can become a high-capacity, fast-discharge and non-dissolvable battery cathode material. According to this study, in sodium ion batteries, this polymer is superior to current polymers and inorganic cathodes in capacity transfer and capacity retention, and this performance is not lagging in multivalent magnesium ion and aluminum ion batteries. too much.
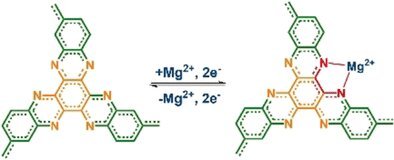
Scientists have found that hexaazatriphthalene (HATN) is a very suitable cathode material, and this compound has been tested in lithium batteries and supercapacitors to prove that it can become a high energy density cathode, fast Insert into lithium ions. However, like most organic materials, HATN will dissolve in the electrolyte, causing the cathode to become unstable during the charge and discharge cycle. The scientists explained that the key now is to stabilize the structure of the material by allowing the connection between single molecules, and the result is an organic polymer called polymeric HATN or PHATN, which enables sodium, aluminum and magnesium ions to react quickly. Power and high capacity.
After assembling the battery, the scientists tested the PHATN cathode with a high concentration electrolyte and found that non-lithium ions have excellent electrochemical performance. The sodium battery can work at a high voltage of up to 3.5V. Even after 50,000 cycles, its capacity can still be maintained above 100 milliampere hours per gram.
Researchers believe that this type of polymerized p-diaza cathode (p-diazabenzene is a HATN-based organic substance, an aromatic hydrocarbon nitrogen-rich organic substance with a fruity flavor) can achieve environmental protection, high energy density, The next generation rechargeable battery with fast and ultra-stable charge and discharge. (Author: Yuqiu Yun)
Medical Uv Lamp,Safe Uv Light,Uv Lamp Power,360 Nm Uv Light
Guangdong Kingrate Optoelectronic Technology Co., Ltd. , https://www.kingrateuv.com