In soils P may exist in many different forms. In practical terms, however, P in soils can be thought of existing in 3 "pools":
-
solution P
-
active P
-
fixed P
The solution P pool is very small and will usually contain only a fraction of a pound of P per acre. The solution P will usually be in the orthophosphate form, but small amounts of organic P may exist as well. Plants will only take up P in the orthophosphate form. The solution PS pool is important because it is the pool from which plants take up P and is the only pool that has any measurable mobility. Most of the P taken up by a crop during a growing season will probably have moved only an inch or less through the soil to the roots. A growing crop would quickly deplete the P in the soluble P pool if the pool was not being continuously replenished.
The active P pool is P in the solid phase which is relatively easily released to the soil solution, the water surrounding soil particles. As plants take up phosphate, the concentration of phosphate in solution is decreased and some phosphate from the active P pool is released. Because the solution P pool is very small, the active P pool is the main source of available P for crops. The ability of the active P pool to replenish the soil solution P pool in a soil is what makes a soil fertile with respect to phosphate. An acre of land may contain several pounds to a few hundred pounds of P in the active P pool. The active P pool will contain inorganic phosphate that is attached (or adsorbed) to small particles in the soil, phosphate that reacted with elements such as calcium or aluminum to form somewhat soluble solids, and organic P that is easily mineralized. Adsorbed phosphate ions are held on active sites on the surfaces of soil particles. The amount of phosphate adsorbed by soil increases as the amount of phosphate in solution increases and vice versa (Figure 2). Soil particles can act either as a source or a sink of phosphate to the surrounding water depending on conditions. Soil particles with low levels of adsorbed P that are eroded into a body of water with relatively high levels of dissolved phosphate may adsorb phosphate from the water, and vice versa.
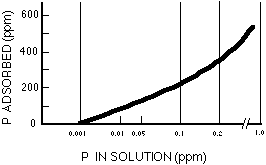

Figure 2. Relationship between P absorbed by soil and P in solution.
The fixed P pool of phosphate will contain inorganic phosphate compounds that are very insoluble and organic compounds that are resistant to mineralization by microorganisms in the soil. Phosphate in this pool may remain in soils for years without being made available to plants and may have very little impact on the fertility of a soil. The inorganic phosphate compounds in this fixed P pool are more crystalline in their structure and less soluble than those compounds considered to be in the active P pool. Some slow conversion between the fixed P pool and the active P pool does occur in soils.

