Hydrogen has extremely high specific energy, and the combustion product is only water, so it is regarded as a clean energy carrier. Electrolyzed water, that is, electrolysis of water to generate hydrogen and oxygen, is a clean method of hydrogen production. In order to promote the hydrogen evolution reaction of electrolyzed water (2H + + 2e- → H2) and achieve a higher reaction rate at a lower overpotential, it is often necessary to use an efficient catalyst. Under acidic conditions, platinum-based metals such as Pt and Ir are the most effective hydrogen evolution catalysts. In order to more effectively use platinum-based metals such as Pt and Ir, two main strategies have been proposed: 1. Reduce the dimension of the precious metal catalytic material to increase the specific surface area; 2. Alloy precious metal and non-precious metal elements to reduce the precious metal Dosage. Although combining these two strategies is more promising to obtain catalytic materials with higher activity and lower precious metal usage, alloy catalysts based on non-precious metal elements are likely to cause insufficient stability due to corrosion under acidic conditions.
Amorphous alloy (also called metallic glass) is a new type of multi-component alloy material with a unique disordered atomic structure. Unlike crystalline materials where atoms are in equilibrium, metastable amorphous alloys exhibit many excellent mechanical and physicochemical properties, attracting widespread attention in many fields such as materials science and condensed matter physics. The team of Liu Yanhui and Wang Weihua of the Institute of Physics, Chinese Academy of Sciences / National Research Center for Condensed Matter Physics in Beijing recently introduced the concept of genetic engineering of materials in the field of amorphous alloys, developed unique high-throughput experimental methods, and realized the efficient exploration of new materials for amorphous alloys. Successfully developed Ir-Ni-Ta high temperature amorphous alloy new material system [Nature, 99, 569 (2019)]. In addition to exhibiting excellent mechanical properties, this new material system also contains rich, yet to be explored functional characteristics. For example, Ir-Ni-Ta amorphous alloy has the characteristics of strong corrosion resistance and can be immersed in aqua regia for several months without being corroded. Combined with the catalytic activity of Ir element, this material system is expected to solve the problem of insufficient stability of alloy catalysts based on non-noble metals under acidic conditions.
Recently, the team carried out research on the catalytic properties of Ir-Ni-Ta amorphous alloy. The doctoral student Wang Zijian of the team was prepared by ion beam sputtering deposition method under the joint guidance of researchers Liu Yanhui, Wang Weihua and associate professor Ge Zhibo of Southwest Petroleum University Ir25Ni33Ta42 amorphous alloy film tested the catalytic activity and stability of the hydrogen evolution reaction of the new material system Ir-Ni-Ta under acidic conditions.
The thickness of Ir25Ni33Ta42 amorphous alloy thin film is 15 nm, and the loading of precious metal Ir is about 8.14 μg cm-2. It is worth noting that the surface of the film shows flatness close to the atomic level, which is extremely important for evaluating the authentic catalytic activity of the material. In the 0.5 M H2SO4 environment, the Ir25Ni33Ta42 amorphous alloy thin film can drive a current density of 10 mA cm-2 with an overpotential of only 99 mV. Although this value is higher than that of Pt (46 mV) and Ir (59 mV) films under the same conditions, it is much lower than that of phosphide films such as CoP (202 mV). The Tafel slope of Ir25Ni33Ta42 amorphous alloy film is 35 mV dec-1, which is similar to that of Pt (28 mV dec-1) and Ir (30 mV dec-1) films. After 1000 cyclic voltammetry scans, the catalytic activity of the Ir25Ni33Ta42 amorphous alloy film did not change; the change in overpotential was detected at a constant current density of 10 mA cm-2. After 10 hours of testing, the overpotential increased Only 50 mV. In contrast, the increase in overpotential of Pt and Ir films after 10 hours of testing was as high as 250 and 200 mV, respectively. This shows that Ir25Ni33Ta42 amorphous alloy thin film has higher catalytic stability than Pt and Ir.
Compared with the reported amorphous alloy catalysts, Ir25Ni33Ta42 amorphous alloy thin films have both low overpotential and Tafel slope. What is particularly important is that its catalytic activity does not come from a complex surface structure or high precious metal loading, but the performance of this certificate. Calculations show that the number of hydrogen molecules produced by Ir25Ni33Ta42 amorphous alloy at a single active site per unit time is much higher than that of transition metal sulfides and phosphides, and it is comparable to other precious metal-containing catalysts. The excellent catalytic performance of Ir25Ni33Ta42 amorphous alloy is mainly attributed to its alloy system and amorphous structure. This work not only provides a new system for hydrogen evolution reaction catalytic materials, but also helps to design and develop multi-component alloys for other heterogeneous catalytic reactions.
The research work was supported by the National Outstanding Youth Science Foundation (51825104), the Hundred Talents Program of the Chinese Academy of Sciences, the Chinese Academy of Sciences Frontier Science Key Research Program (QYZDY-SSW-JSC017), the Chinese Academy of Sciences' Strategic Leading Science and Technology Project (XDB30000000), the National Key Research and Development Program (2018YFA0703601), and the country Support from the Natural Science Foundation (11790291, 61888102), etc.
Related research results were recently published online in "Advanced Materials" (Advanced Materials).
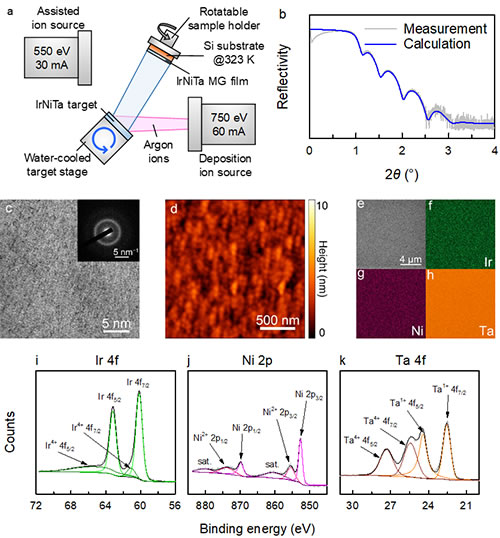
Figure 1. Preparation and characterization of Ir25Ni33Ta42 amorphous alloy thin film
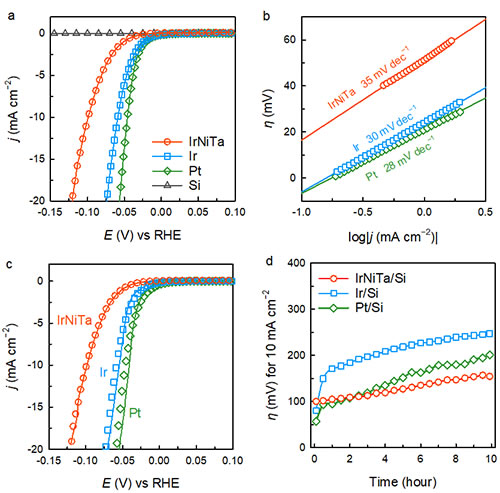
Figure 2. The catalytic activity and stability of Ir25Ni33Ta42 amorphous alloy film for hydrogen evolution reaction
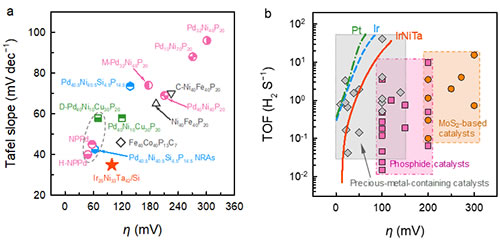
Figure 3. Comparison of catalytic performance of Ir25Ni33Ta42 amorphous alloy film and other materials for hydrogen evolution reaction
Ningbo Wason Lighting Technology Co.,Ltd , https://www.wasonlight.com