The P cycle is similar to several other mineral nutrient cycles in that P exists in soils and minerals, living organisms, and water. Although P is widely distributed in nature, P is not found by itself in elemental form. Elemental P is extremely reactive and will combine with oxygen when exposed to the air. In natural systems like soil and water, P will exist as phosphate, a chemical form in which each P atom is surrounded by 4 oxygen (O) atoms. Orthophosphate, the simplest phosphate, has the chemical formula PO4-3. In water, orthophosphate mostly exists as H2PO4- in acidic conditions or as HPO42- in alkaline conditions.
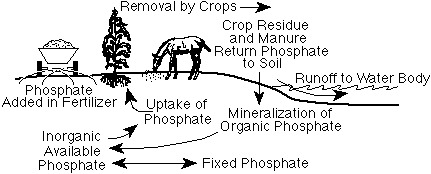

Figure 1. The phosphorus cycle.
Phosphate is taken up by plants from soils, utilized by animals that consume plants, and returned to soils as organic residues decay in soils (Figure 1). Much of the phosphate used by living organisms becomes incorporated into organic compounds. When plant materials are returned to the soil, thisorganic phosphate will slowly be released as inorganic phosphate or be incorporated into more stable organic materials and become part of the soil organic matter. The release of inorganic phosphate from organic phosphates is called mineralization and is caused by microorganisms breaking down organic compounds. The activity of microorganisms is highly influenced by soil temperature and soil moisture. The process is most rapid when soils are warm and moist but well drained. Phosphate can potentially be lost through soil erosion and to a lesser extent to water running over or through the soil.
Many phosphate compounds are not very soluble in water; therefore, most of the phosphate in natural systems exists in solid form. However, soil water and surface water (rivers and lakes) usually contain relatively low concentrations of dissolved (or soluble) phosphorus. Depending on the types of minerals in the area, bodies of water usually contain about 10 ppb or more of dissolved P as orthophosphate. Water bodies may also contain organic P and phosphate attached to small particles of sediment. Total phosphorus in water is all of the phosphorus in solution regardless of its form and is often the form reported in water quality studies. Algal available or bioavailable phosphorus is P that is estimated to be available to organisms like algae that are present in a lake or river. This is usually estimated by a chemical test which is designed to measure the dissolved P and the particulate P that are easily available. This is a measure of the P that is of immediate concern to water quality.
The word phosphorus or P refers to the element and is also used as a general term when a particular chemical form of P is not being designated. For example, the total P content of a soil or plant material is usually expressed as percent P. However, fertilizer analyses are usually reported as percent P2O5. The phosphate form (P2O5) is a chemical produced during fertilizer analysis, but does not exist in either fertilizers or soils.