Recently, the research group Li Yue of the Department of Micro-nano Technology and Devices of the Institute of Solid State Physics, Chinese Academy of Sciences, Hefei Institute of Physical Research, made progress in the construction and application of electrocatalytic hydrogen evolution electrode materials. The relevant research results were published on Nanoscale. Selected as the current Inside back cover.
Hydrogen as a pollution-free ecological clean energy has attracted much attention. Hydrogen production from electrolyzed water is an important means to achieve industrialization and cheap hydrogen production. The premise of realizing industrial hydrogen production from electrolyzed water is to obtain electrocatalytic hydrogen evolution electrode material with high catalytic activity. It is well known that precious metal-based catalysts represented by platinum have the highest efficiency of hydrogen production by electrolysis under electrocatalytic conditions; however, their high prices and scarce reserves hinder their large-scale industrialization. Therefore, the development of non-precious metal catalysts with high catalytic performance to replace precious metal catalysts has important practical significance and wide application prospects for realizing large-scale hydrogen production from electrolyzed water.
Among many non-precious metal hydrogen evolution catalysts, transition metal phosphides have attracted much attention due to their low cost and high electrocatalytic hydrogen evolution activity. In particular, a three-dimensional nano-array catalyst constructed by phosphide doped with elements such as molybdenum (Mo), cobalt (Co), and nickel (Ni) causes the hydrogen on the surface of the solid catalyst due to the regulation of the electronic structure of the phosphide by the doping element. The atomic adsorption properties are degraded, which makes its hydrogen evolution performance comparable to that of platinum. However, in actual applications, the catalytic activity of non-noble metal catalysts is greatly affected by the pH environment in which they are located, and the stability of non-precious metal catalysts needs to be further improved.
In view of this, Li Yue's group used NiMoO4 nanowires as precursors, and after subsequent annealing phosphating process, the preparation of Mo-doped Ni2P three-dimensional nanoarrays was realized. The Mo-Ni2P nanowire array electrocatalyst has excellent electrocatalytic activity under different pH environments (acidity, neutrality, and basicity) and reaches a current of 10 mA/cm2 in an electrolyte solution with pH of 0, 7, and 14. The required overpotentials for density are 64, 84, and 78 mV, respectively (Figure 2). This indicates that the catalyst system has high electrocatalytic activity in a wide pH environment range; the catalyst system has high electrochemical stability over the entire pH range. The preparation of the Mo-Ni2P nanowire array electrocatalyst will provide a new approach for the design and application of novel, efficient, stable, and inexpensive non-precious metal hydrogen evolution electrocatalysts.
The research work was funded by the cross-team project of the Chinese Academy of Sciences and the National Natural Science Foundation.

Figure 1. Inner cover diagram of hydrogen production from electrolysis of water over the whole pH range of Mo-Ni2P three-dimensional nanowire arrays.
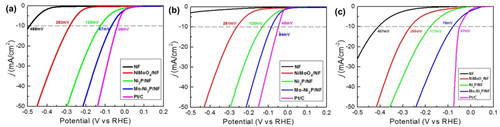
Figure 2. Linear scan voltammograms of Mo-Ni2P three-dimensional nanowire arrays under (a) acidic; (b) neutral; (c) alkaline conditions.
Pvc Self-adhesive Floor Stickers
pvc self adhesive flooring,peel and stick vinyl floor stickers,removable vinyl floor stickers,PVC floor glue floor sticker,pvc adhesive floor wallpaper
JIANGSU ARTSTYLE DECORATION MATERIALS CO..LTD , https://www.artstyledecor.com