With the development of electric vehicles and large-scale energy storage, existing lithium-ion battery systems have begun to fail to meet the growing demand, and there is an urgent need to develop battery systems with higher energy density. In many battery material systems, metal lithium anodes have the lowest potential and the highest theoretical specific capacity, and are regarded as the ultimate choice of battery anode materials. However, lithium metal will react with the liquid electrolyte, and lithium dendrites will be generated as the battery cycles, resulting in a lower cycle life and poor safety of the battery, which seriously hinders the large-scale application of lithium metal batteries. The all-solid-state battery replaces the liquid electrolyte with a non-flammable solid electrolyte with a certain rigidity, and some solid-state electrolytes show good compatibility with metallic lithium, so the all-solid-state battery is considered to be expected to achieve both high energy density and high safety. However, there is relatively little research work on the safety of all solid-state batteries, and the understanding of the safety of all solid-state batteries is not deep enough.
Under the guidance of researchers Yu Xiqian and Li Hong, Dr. Chen Rusong, group E01 of the Clean Energy Key Laboratory of the Institute of Physics, Chinese Academy of Sciences / National Research Center for Condensed Matter Physics, Beijing, studied a few main types of techniques using adiabatic accelerated calorimetry (ARC) The thermal stability of the oxide solid electrolyte and metal lithium was found to be different from the garnet type solid electrolyte LLZO, and other solid electrolytes contacted with the metal lithium in different degrees of thermal runaway during heating and temperature rise. The team further cooperated with Professor Yifei Mo of the University of Maryland and combined with theoretical calculations to reveal the internal mechanism of thermal runaway of solid electrolyte and lithium metal at high temperature. The research result was recently published in "Joule" (Joule, 2020, DOI: 10.1016 / j.joule.2020.03.012) entitled The Thermal Stability of Lithium Solid Electrolytes with Metallic Lithium.
The research team selected four mainstream oxide solid electrolyte materials as research objects, namely Li1.5Al0.5Ge1.5 (PO4) 3 (LAGP) and Li1.4Al0.4Ti1.6 with sodium super ion conductor structure (NASICON) (PO4) 3 (LATP), Li3xLa2 / 3-xTiO3 (LLTO) with perovskite structure, and Li6.4La3Zr1.4Ta0.6O12 (LLZO) with garnet structure. Adiabatic accelerated calorimeter was used to quantitatively determine the characteristic temperature, heat production rate and heat production of thermal runaway reaction between them and metallic lithium. The results show that both LAGP, LATP and lithium metal have obvious thermal runaway during heating and accompanied by a large amount of heat release, LLTO has a slight heat release phenomenon, while LLZO has no obvious heat generation. Then the research team combined with DFT thermodynamic calculations and found that the heat generated by the chemical reaction between the solid electrolyte and the lithium metal is insufficient to cause thermal runaway. The cause of the thermal runaway is likely to be the heat generated by the chemical reaction of the interface when the solid electrolyte is in contact with the lithium metal. The solid electrolyte material itself decomposes to generate oxygen, and the oxygen further reacts with metal lithium to cause intense heat generation. The research team further confirmed this conjecture by performing accurate XRD phase analysis of the reaction products. The above research indicates that some solid electrolyte materials widely considered to have high structural thermal stability may still undergo thermal runaway reactions at high temperatures after contact with metal lithium anodes. This shows that the thermal stability of the material itself does not represent its overall thermal stability in the battery, and the reaction characteristics of the solid electrolyte and other active materials and inactive materials in the battery need to be considered.
This work explains the complexity of battery safety factors, and emphasizes the necessity and urgency of research on the safety of all solid-state batteries. In addition to safety, the chemical / electrochemical stability and mechanical stability caused by the replacement of the liquid electrolyte of the lithium-ion battery by the solid electrolyte make the current electrochemical performance of the all-solid battery still far from the actual application . The research team recently systematically summarized and discussed the topic of Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interface (Chemical Reviews, 2019, DOI: 10.1021 / acs.chemrev.9b00268) in the journal Chemical Reviews. Various interface stability problems in all solid-state batteries. Whether all-solid-state batteries can eventually be put into large-scale practical use, there are still a lot of basic scientific problems that need to be studied urgently.
Related work was supported by the Ministry of Science and Technology's Key R & D Program (2016YFB0100100), the Fund's Excellent Youth Fund (51822211) and the National Natural Science Foundation of China (U1932220).
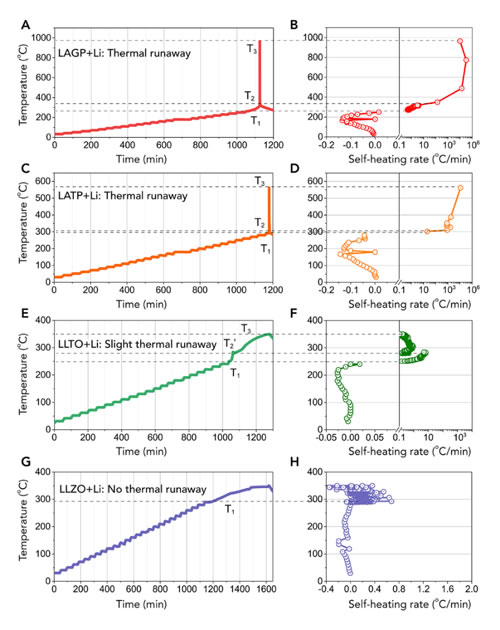
Figure 1. ARC test results of four oxide solid electrolytes in contact with metallic lithium.
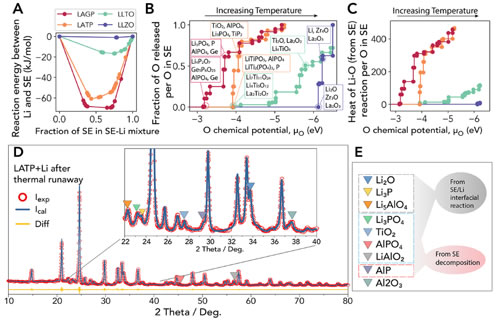
Figure 2. a) Chemical reaction energy of oxide solid electrolytes with different mixing ratios reacting with lithium metal; b) Comparison of the ease of decomposition of oxide solid electrolytes to generate oxygen; Comparison of heat; d) Phase analysis of residual samples after thermal runaway of LATP / Li; e) Attribution of each substance in XRD to chemical reaction products and solid electrolyte decomposition products of solid electrolyte / lithium interface
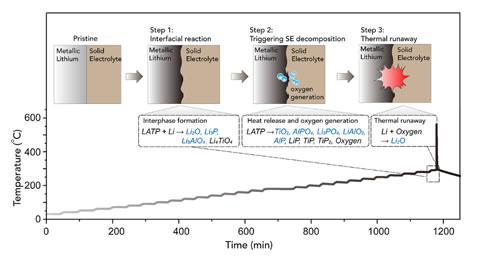
Figure 3. Taking LATP as an example to illustrate the thermal runaway reaction mechanism of oxide solid electrolyte-metallic lithium.
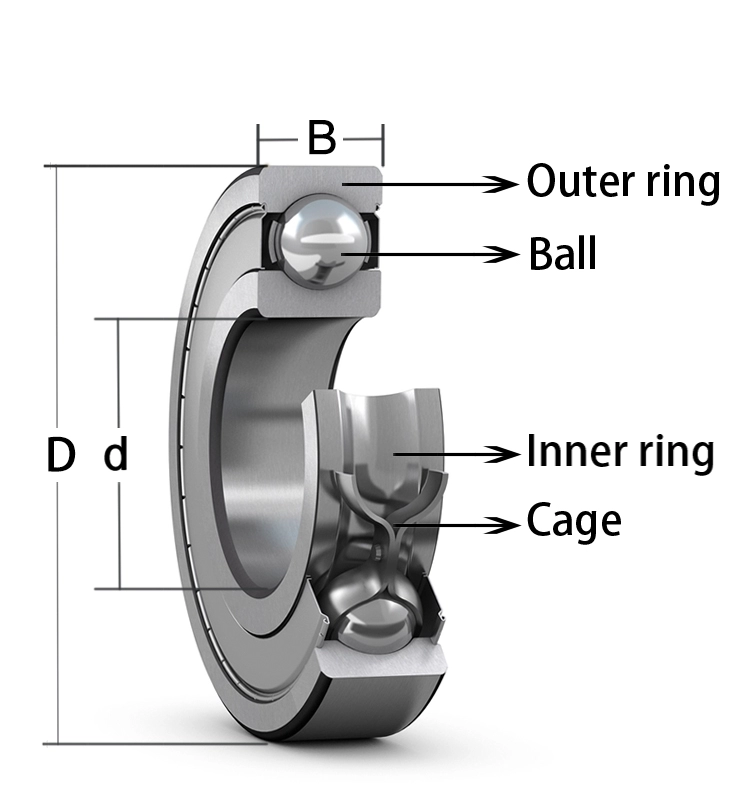
Deep Groove Ball Bearings
Deep groove ball bearings The out looks of them are nearly the same,the only different is the size,you may need other models please check the following table for reference.are the most widely used bearing type and are particularly versatile.
They have low friction and are optimized for low noise and low vibration which enables high rotational speeds.
They accommodate radial and axial loads in both directions, are easy to mount, and require less maintenance than other bearing types.
Deep groove ball bearings are divided into the following series: 6000series, 6100series, 6200series, 6300series, 6400 series, 6700 series, 6800 series,6900 series,16000 series etc.
|
Model |
d(mm) | D(mm) | B(mm) |
|
6000ZZ |
10 | 26 | 8 |
| 6001ZZ | 12 | 28 | 8 |
| 6002ZZ | 15 | 32 | 9 |
| 6003ZZ | 17 | 35 | 10 |
| 6004ZZ | 20 | 42 | 12 |
| 6005ZZ | 25 | 47 | 12 |
| 6006ZZ | 30 | 55 | 13 |
| 6007ZZ | 35 | 62 | 14 |
| 6008ZZ | 40 | 68 | 15 |


We manage in all kinds of ball bearings and roller bearings,such as
1.Deep Groove Ball Bearing
2.Self-aligning Ball Bearing
3.Cylindrical Roller Bearing
4.Spherical Contact Bearing
5.Angular Contact Bearing
6.Tapered Roller Bearing
7.Thrust Ball Bearing
8.Insert Bearing
Package
1.Small bearings|:plastic tube+carton+pallet
2.Medium bearings:PE bag+box+carton+pallet
3.Large bearings:PE film & paper winding+carton+pallet
4.According to the customer's requirements
High Ball Bearing,Miniature Ball Bearing,Deep Groove Ball Bearing,Deep Ball Bearing
Shijiazhuang Longshu Mechanical & Electrical Equipment Trading Co., Ltd. , https://www.longsbearings.com