The phosphate in fertilizers and manure is initially quite soluble and available. Most phosphate fertilizers have been manufactured by treating rock phosphate (the phosphate-bearing mineral that is mined) with acid to make it more soluble. Manure contains soluble phosphate, organic phosphate, and inorganic phosphate compounds that are quite available. When the fertilizer or manure phosphate comes in contact with the soil, various reactions begin occurring that make the phosphate less soluble and less available. The rates and products of these reactions are dependent on such soil conditions as pH, moisture content, temperature, and the minerals already present in the soil.
As a particle of fertilizer comes in contact with the soil, moisture from the soil will begin dissolving the particle. Dissolving of the fertilizer increases the soluble phosphate in the soil solution around the particle and allows the dissolved phosphate to move a short distance away from the fertilizer particle. Movement is slow but may be increased by rainfall or irrigation water flowing through the soil. As phosphate ions in solution slowly migrate away from the fertilizer particle, most of the phosphate will react with the minerals within the soil. Phosphate ions generally react by adsorbing to soil particles or by combining with elements in the soil such as calcium (Ca), magnesium (Mg), aluminum (Al), and iron (Fe), and forming compounds that are solids. The adsorbed phosphate and the newly formed solids are relatively available to meet crop needs.
Gradually reactions occur in which the adsorbedphosphate and the easily dissolved compounds of phosphate form more insoluble compounds that cause the phosphate to be become fixed and unavailable. Over time this results in a decrease in soil test P. The mechanisms for the changes in phosphate are complex and involve a variety of compounds. In alkaline soils (soil pH greater than 7) Ca is the dominant cation (positive ion) that will react with phosphate. A general sequence of reactions in alkaline soils is the formation of dibasic calcium phosphate dihydrate, octocalcium phosphate, and hydroxyapatite. The formation of each product results in a decrease in solubility and availability of phosphate. In acidic soils (especially with soil pH less than 5.5) Al is the dominant ion that will react with phosphate. In these soils the first products formed would be amorphous Al and Fe phosphates, as well as some Ca phosphates. The amorphous Al and Fe phosphates gradually change into compounds that resemble crystalline variscite (an Al phosphate) and strengite (an Fe phosphate). Each of these reactions will result in very insoluble compounds of phosphate that are generally not available to plants. Reactions that reduce P availability occur in all ranges of soil pH but can be very pronounced in alkaline soils (pH > 7.3) and in acidic soils (pH < 5.5). Maintaining soil pH between 6 and 7 will generally result in the most efficient use of phosphate (Figure 3).

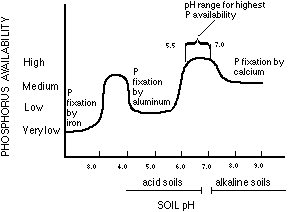
Figure 3. The availability of phosphorus is affected by soil pH.
Adding to the active P pool through fertilization will also increase the amount of fixed P. Depleting the active pool through crop uptake may cause some of the fixed P to slowly become active P. The conversion of available P to fixed P is partially the reason for the low efficiency of P fertilizers. Most of the P fertilizer applied to the soil will not be utilized by the crop in the first season. Continued application of more P than the crops utilize increases the fertility of the soil, but much of the added P becomes fixed and unavailable.
Most fine-to medium-textured soils have large capacities to hold phosphate by adsorption and precipitation. Occasionally the question of how much phosphate a soil can hold is asked, especially when high loading rates of P are expected or have occurred. Soils differ in their phosphate holding capacity. Fine-textured soils can generally hold hundreds of pounds of phosphate per acre. while coarse-textured soils can generally hold much less phosphate due to the more inert character of sand particles as compared to clay particles. In addition the subsoil of many soils often has an even greater capacity to hold phosphate than does the corresponding surface soil. However, an important aspect of the ability of a soil to hold phosphate is that a soil cannot hold increasing amounts of phosphate in the solid phase without also increasing soil solution phosphate (Figure 2). Increased amounts of phosphate in solution will potentially cause more phosphate to be lost to water running over the soil surface or leaching through the soil. Loading soils with very high levels of phosphate will generally not hurt crops but may result in increased phosphate movement to nearby bodies of water.