Synthetic diamond abrasives are made into various abrasive tools and are widely used in manufacturing, such as aerospace, automotive, marine, hydraulic, engine, bearing, optical components and many other fields. Due to its high thermal conductivity, good chemical inertness, good optical transparency (from UV to IR) and corrosion resistance, it can be used as a field emitter in the electronics industry as a chemical and biosensor for DNA and protein chips. The electrode material is used for electrocatalytic reaction, and is used as a highly thermally conductive material for electronic packaging materials and the like. However, the diamond surface is also chemically inert and difficult to combine with many materials, limiting its range of applications.
In order to improve the surface properties of diamond and improve its ability to bond with other materials, researchers have modified its surface by different methods, such as metallization of diamond surface, surface coupling agent or surfactant treatment and diamond surface function. To enhance or extend the performance and scope of its use. We review the modification of diamond surfaces and provide references for their application and research.

Diamond
Metallization of the diamond surface
Covering the surface of the diamond with a metal or alloy material is referred to as metallization of the diamond surface. The covering method can be classified according to whether interface reaction occurs: (1) physical vapor deposition, electroless plating, electroplating, etc.; (2) methods for interfacial reaction include chemical vapor deposition, vacuum micro-evaporation, and salt bath. Plating and so on.
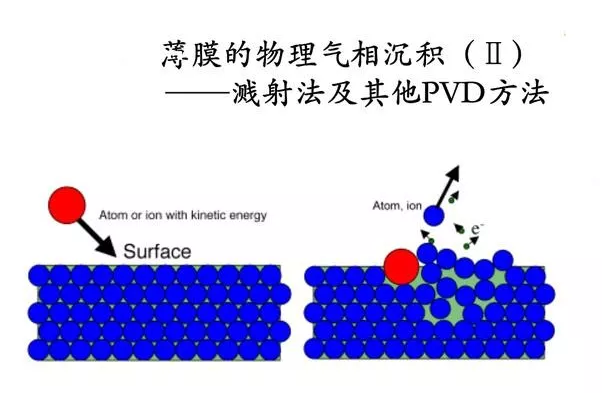
Physical vapor deposition of thin films
Physical vapor deposition is a process of depositing a solid or liquid film-forming material into a matrix by gas phase atoms, molecules, and ions; chemical vapor deposition is a technique in which two or more kinds of gaseous raw materials are deposited on a surface of a substrate by a chemical reaction. Metallization of Ti, Cr, W and their alloys on the diamond surface can be achieved by both physical and chemical vapor deposition. However, the reaction phases of the two methods are difficult to penetrate into the inside of the diamond particles, and there is a problem that the diamond is covered a small amount and the production cost is high, and it is difficult to realize industrial application.
The salt bath plating is to add a metal powder such as Ti or Cr to the salt of the chloride, and the salt bath is treated at a high temperature for 1-2 hours, and the diamond surface reacts in the molten metal to form a carbide layer. The salt bath plating temperature is too high, the diamond surface is prone to thermal damage, and the diamond separation process is complicated and the cost is relatively high.
Electroless plating, electroplating, and vacuum micro-evaporation plating can realize industrialized large-scale diamond surface metallization production. In industrial applications, metal plating is generally performed first, and then plating is thickened by electroplating or the like.
Diamond electroless plating refers to the process of reducing the deposition of metal ions on the surface of diamond by using a reducing agent in the plating solution. Liu Shimin et al studied the process of electroless Ni-P plating on the surface of synthetic diamond (particle size code 80/100). It was found that the microstructure of the P mass fraction greater than 8% is amorphous, and the heat treatment can make the Ni-P coating microstructure amorphous. Change to a crystalline state. Zhang Fenglin et al. electrolessly plated Ni and Cu on the surface of diamond (particle size code 40/50). The coating can improve the high temperature thermal erosion resistance of diamond, and the effect of Ni coating is more obvious than that of Cu coating. Yao Huai et al. studied the effect of Ni-P coating on the surface of diamond micropowder (average particle size 50-70um) when the pH value was 11-14. When the pH of the plating solution is 13, the deposition of the coating is dense and complete; when the pH is less than 13, the coating is incomplete; when the pH is greater than 13, the coating will fall off.
On the basis of Ni-P plating, in order to improve the coating performance, the researchers also studied the ternary coating of diamond. For example, in order to improve the corrosion resistance and hardness of diamond, Xiang Dong et al. electrolessly plate Ni-WP on the surface of diamond. After high temperature treatment, the coating forms WC with diamond. To improve the tensile strength and oxidation resistance of diamond particles, Duan Longchen Ni-WB is electrolessly plated on the diamond surface; in order to improve the heat resistance of the diamond particles, Han Kaixin electrolessly deposits Ni-Mo-P on the diamond surface.
Electroless plating is performed by roughening, activating, sensitizing, etc. the diamond surface to make the diamond conductive. The nickel plating layer of electroless nickel plating is thin, and nickel plating is performed by barrel plating to make the thickness of the plating on the diamond surface several tens of micrometers.

Vacuum decompression evaporation distillation extraction rotary evaporator
Vacuum micro-evaporation refers to the process of controlling the reaction temperature and forming a new substance between diamond and certain metals to form a coating under the condition that the diamond interface can form a compound but does not cause thermal damage to the diamond.
The method can improve the amount of single metal deposition, has good metallization effect, low cost, and can realize diversification of plating layers, such as Ti-Cr, Ti-W, Ti-Mo alloy, and the like. Wang Mingzhi and others used this method to deposit Ti on the surface of diamond. It was found that a carbide layer appeared between the Ti coating and the diamond, and the bonding between the coating and the diamond was firm. Wang Yanhui and others have improved the vacuum micro-evaporation plating technology. The surface of the diamond is coated with titanium. The plating temperature is as low as 650 ° C. Each time the diamond can be plated with more than 20,000 ct, and the cost of titanium plating is less than 0.01 yuan / ct. The titanium-plated diamond produced by the vacuum micro-evaporation plating technology has been widely used, the tool life is prolonged, and the processing efficiency can be greatly improved by 30%-120%.
Coupling agent or surfactant treatment
The coupling agent is a substance having two functional groups having different properties, and its molecular structure contains two groups having different chemical properties. One is a pro-inorganic group that is susceptible to chemical reaction with diamond; the other is an organophilic group that chemically reacts with synthetic organic compounds such as synthetic resins or other polymers or generates hydrogen bonds.
Chu Yaqing and others used silane coupling agent (γ-MPS) to modify the surface of ultra-fine diamond, which greatly improved the mechanical properties of the composite resin. Gao wave uses silane coupling agent (KH-570) to modify the surface of diamond micropowder, which makes the polycarbonate composite resin toughen. Ye Xiaochuan et al. modified the diamond surface with a silane coupling agent, and combined the modified diamond with polyimide, heat-resistant phenolic and modified phenolic binder to form a grinding wheel, which improved the bonding state of diamond and resin and improved the resin. For the holding power of diamond, the grinding ratio of the polyimide resin grinding wheel is increased by 109.9%. Lu Jing and others used KH-570 to modify the surface of ultra-fine diamond to improve the holding power of the bonding agent to diamond. Bandung and other modified KH-550 diamond surface to improve its dispersion in aqueous alcohol solution and toluene solution, the dispersion effect of modified diamond in toluene solution is better than its dispersion in aqueous alcohol solution.
The surfactant contains hydrophilic and/or lipophilic groups, which can be aligned on the surface of the solution to improve the dispersibility of the diamond in the solution system. Xu Xiangyang modified the nanodiamond with surfactant (STA-10, CR-0704) and dispersant (PEA) to improve its dispersibility in aqueous and non-polar solvents. Zhang Wei et al. modified the Ti-plated diamond surface with a surfactant (sodium dodecyl sulfate, cetyltrimethylammonium bromide) to improve the performance of the diamond-nickel composite coating. Chen Jing et al. To improve the suspension of diamond in polyamide-imide resin solution and its compatibility with resin, respectively, the surface of diamond was modified with silane coupling agent (KH-550) and polyethyleneimine surfactant respectively. The results show that the modification effect of KH-550 is better than that of polyethyleneimine.
Functionalization of the diamond surface
For surface modification of diamond films and nanodiamonds, researchers have tried various methods by introducing different functional groups on the surface of the diamond, such as halogen, amino, oxy (carbonyl, carboxyl) and other functional groups. On this basis, biologically active macromolecules, polymer matrices, and the like can be directly attached to the diamond.
Before the introduction of other functional groups, the diamond needs to introduce a hydrogen terminal on the surface thereof, because the hydrogen-terminated surface is easier to introduce the active group, so that the functionalization of the diamond surface is relatively easy. For the diamond film, it is generally heated to 800-1000 ° C under a hydrogen atmosphere, or treated with a hydrogen plasma to reduce the surface to a uniform clean reaction surface terminated by hydrogen. For nanodiamonds, the oxygen-containing groups carried on the surface thereof have hydroxyl groups, carboxyl groups, ether bonds, carbonyl groups, etc., and a single functional group containing hydrogen on the surface can be obtained by a reduction reaction. On this basis, further functionalization. These methods mainly include (1) chemical modification; (2) photochemical modification; (3) electrochemical modification; (4) nano metal and metal oxide modification.
(1) Chemical modification
Treatment of diamond with an oxidizing acid solution (such as nitric acid, chromic acid, Fenton's reagent, etc.) removes impurities (graphite and metal) from the diamond surface and forms a CO surface functional group on the diamond surface. The diamond (100) surface mainly forms a carbonyl group. And the ether group functional group, the diamond (111) surface mainly forms a hydroxyl functional group. Carboxylated nanodiamonds can be obtained using hydrogen peroxide, a piranha solution (a mixture of sulfuric acid and hydrogen peroxide). At 250-400 ° C, chlorine replaces the hydrogen on the surface of the diamond film, and the surface of the diamond film forms a reactive site, which is easily reacted with nucleophiles (such as H 2 O, NH 3 , CHF).
(2) Photochemical modification
There are two typical photochemical modification techniques: 1 in the ultraviolet light, the olefin reacts with the diamond surface to produce carbon-carbon bonds; 2 uses various types of organic peroxides to initiate free radical reactions. Photochemical methods can attach the diamond surface to an alkyl chain, carboxylic acid or primary amine group. YANG et al. used the second method to attach the DNA strand to the diamond surface, and the DNA strand was well connected. UV illumination can also be used to activate free radical reactions. For example, MILLER uses this technology to chlorinate the diamond surface to achieve amine or thiolation of the diamond surface. SMENTKOWSKI and other materials form a very stable CF terminal on the surface of the diamond film by photochemical modification. .
(3) Electrochemical modification
The electrochemical modification method comprises: 1 performing anodic polarization in an acid or alkali solution; 2 adding an aromatic diazonium salt to the electrolyte solution, and introducing an aromatic group on the surface of the diamond. Compared with the chemically modified oxidation method, the electrochemical modification method can rapidly achieve oxidation in a wide range; compared with the plasma oxidation method, the oxidation process is the easiest to implement because it does not involve high energy and can avoid thermal damage of the diamond surface. The electrochemical oxidation method is used to form a C=O bond on the surface of the diamond, which is prepared into a diamond film electrode, which can improve the detection precision and selectivity. Diamond thin film electrodes have been applied in electrical analysis and electrochemical degradation of organic pollutants.
(4) Metal and metal oxide modification
Nano-electronic devices can be prepared by depositing metal particles (such as gold, copper, silver, nickel, platinum, rhodium, and palladium) on the surface of diamond by thermal deposition or potentiostatic electrodeposition. They are used in catalytic reactions, disease diagnosis and treatment, and biology. Sensing and other fields. For example, the diamond/platinum composite electrode not only has good catalytic activity, but also has excellent corrosion resistance and stability, and can be applied to an electrochemical energy conversion device (such as a fuel cell); electrodepositing nano gold to a diamond surface. The membrane electrode has the catalytic ability to reduce the O2 reduction reaction in an acidic solution, and the catalytic efficiency is 20 times that of the gold electrode under the same conditions; after the copper and nickel are deposited on the surface of the nano-diamond, the electrocatalytic activity of glucose is improved; The ruthenium or hydrated cobalt oxide is deposited on the surface of the diamond to form a catalytic electrode, which can improve the reduction yield of carbon dioxide to carbon monoxide. In this way, it can reduce carbon dioxide emissions and provide technical support for the use of carbon dioxide as a chemical synthetic raw material.
Conclusion and recommendations
(1) The metallization method of the surface of the synthetic diamond includes physical vapor deposition, chemical vapor deposition, electroless plating, electroplating, vacuum micro-evaporation plating, salt bath plating, and the like. Among them, mature industrialization methods include electroless plating, electroplating, and vacuum micro-evaporation plating. Mainly used in diamond abrasives and abrasives as well as electronic packaging materials.
(2) Coupling agent or surfactant treatment of synthetic diamond to improve the performance of the interface between the organic system and the diamond. It is used in the dispersion of diamond powder and the manufacture of resin abrasives.
(3) Functional modification of the diamond surface is diamond film and nano-diamond. Products are mainly used in electrochemical, biosensor and electrode materials.
1. Elegant circular high-power underwater LED luminaire for underwater landscape lightings.
2. Cree or Lumileds high power leds in whites, mono color and RGB with various beam angle options.
3. High brightness contrast level for smooth and consistent color change effect.
4. Stainless steel construction and clear tempered glass cover.
5. IP68 rated and operate to a depth up to 5 meters.
6. Stainless steel mounting bracket that can rotate and tilt up to 180° for easy and fast installation and light projection adjustment.
7. DC12V input or constant current input option.
Underwater Lights,Underwater LED Lights,Waterproof Underwater Lights,Underwater Pool Lights,Multi-Color Underwater Lights,RGB Underwater Lights
StrongLED Lighting Systems (Suzhou) Co., Ltd. , https://www.strongledcn.com