Activated carbon is a porous material composed of microcrystalline and amorphous carbon with a highly developed pore structure and a large specific surface area. As an excellent adsorbent, activated carbon has been widely used in environmental protection, chemical industry, food processing, hydrometallurgy, pharmaceutical refining, military chemical protection and other fields. Nowadays, various methods for the preparation of activated carbon have been extensively studied in various countries around the world. However, how to optimize various operating parameters is still a cutting-edge study. China is a cotton-producing country with abundant cotton linters. Cotton pulp black liquor is a high-contamination wastewater produced by the chemical fiber plant in the process of producing cotton pulp using cotton linters as raw materials. Its lignin content is high and it is prepared. Excellent raw material for activated carbon. The author used the acid lignin extracted from cotton black liquor as raw material to prepare lignin activated carbon by zinc chloride activation method. The relationship between the operating parameters and the iodine value of each operating parameter was determined by system, and the operation of preparing activated carbon was optimized. Parameters, research to explore the optimal operating conditions for the preparation of high performance activated carbon.
1 Materials and methods
1.1 Raw materials and instruments
Acid lignin, supplied by a paper mill. 101-1 type electric heating constant temperature blast drying box, Shanghai Experimental Instrument Factory; SX2-4-10 high temperature box type electric furnace, Shanghai Pudong Equipment Factory production; HZQ-C type air bath electric shock device, Harbin Dongming Medical Instrument Factory production. All other benchmark reagents were of analytical grade.
1.2 Test methods
1.2.1 Raw material pretreatment.
The acid lignin was finely ground, the impurities were removed, washed with water until neutral, and 1 mol/L of AgNO3 solution was added dropwise without precipitation, and dried and passed through a 200 mesh sieve for use.
1.2.2 Preparation of woody activated carbon.
Weigh 5 g of 200-mesh sieved lignin, add 40% zinc chloride solution, put it at 120 °C for 12 h according to the impregnation ratio, then open the lid and send it to the muffle furnace to raise the temperature. At 200 ° C, then the temperature was raised to a set temperature of 600 ° C at a rate of 10 ° C / min, and activated for 70 min. After pickling and washing with water, the product was dried at 120 ° C for 3 h, ground and crushed through a 200 mesh sieve, and bottled for testing.
1.2.3 Determination of the properties of wood activated carbon.
The ash content is carried out according to GB/T12496.3-1999; the moisture content is carried out according to GB/T 12496.4-1999; the iodine adsorption value is carried out according to GB/T 12496.8-1999; the methylene blue adsorption value is carried out according to GB/T12496.10-1999; The decolorization rate of caramel was carried out in accordance with GB/T12496.9-1999.
2 Results and analysis
2.1 Influencing factors in the stage of Chen release
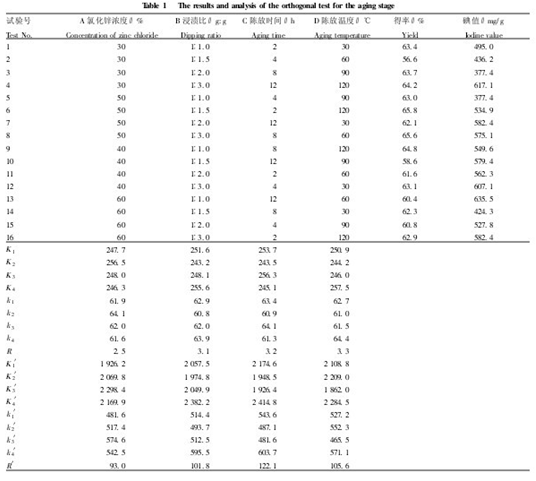
According to the yield and iodine adsorption values, four factors including zinc chloride concentration, impregnation ratio, ageing time and ageing temperature were selected under the conditions of fixed activation time of 50 min, activation temperature of 500 °C and heating rate of 10 °C/min. Four levels were selected and an L16 (45) orthogonal test was performed to examine the primary and secondary effects of each factor. It is shown in Table 1 that the yield is the object of investigation. It can be seen from the range R: the primary and secondary order of each influencing factor is D>C>B>A, ie, the temperature of aging > the time of aging > the ratio of impregnation > the concentration of zinc chloride It shows that the temperature of Chen Fang has the greatest influence on the yield, the time of aging, the ratio of impregnation is the middle, and the concentration of zinc chloride is the smallest. The reason may be that the temperature is too high, the raw material is excessively carbonized, and the amount of carbon is lowered; if the temperature is too low, the carbonization of the raw material is incomplete, and it cannot be deposited in the form of carbon crystallites, and is lost as a gaseous small molecule at a high temperature. In terms of yield, the higher the yield, the better. As shown in Table 1, the optimum process conditions are D4C3B4A2, that is, the temperature of the discharge is 120 ° C, the discharge time is 8 h, the impregnation ratio is 1:3, and the zinc chloride concentration is 50%.
The verification test was carried out according to D4C3B4A2, and the yield was 65.838%, and the iodine adsorption value was 660.9 mg/g. Taking the iodine adsorption value as the object of investigation, it can be seen from the range R' that the primary and secondary order of each influencing factor is C>D>B>A, that is, the time of aging > the temperature of aging > the ratio of impregnation > the concentration of zinc chloride, indicating that The time has the greatest influence on the iodine adsorption value, the temperature of the aging and the impregnation ratio are in the middle, and the concentration of zinc chloride is the smallest. The reason may be: the time is too long, the raw material is excessively carbonized, which is not conducive to the formation of micropores in the activation stage; the time is too short, the carbonization of the raw material is incomplete, the activation stage requires more heat and time to further carbonize, and is not conducive to the formation of pores. As far as the iodine adsorption value is concerned, the higher the iodine adsorption value, the better the adsorption performance of activated carbon. It can be seen from Table 1 that the optimum process condition is C4D4B4A3, that is, the aging time is 12 h, the aging temperature is 120 ° C, and the immersion ratio is 1:3, chlorine. The zinc concentration is 40%. The verification test was carried out according to C4D4B4A3, and the yield was 69.22%, and the iodine adsorption value was 677.13 mg/g. Considering the above situation, the optimum preparation conditions for the Chen-Ping stage are as follows: the age of 12 h, the temperature of 120 ° C, the ratio of impregnation to 1:3, and the concentration of zinc chloride 40%. Two parallel experiments were carried out under the above optimum process conditions to obtain an activated carbon having a yield of 69.0% and an iodine value of 655.88 mg/g.
Table 1 Results and analysis of orthogonal test in the aging stage
2.2 Orthogonal test of various influencing factors in the activation phase
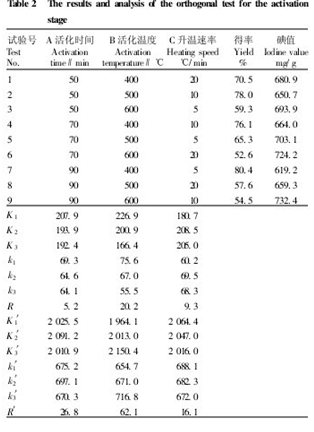
Under the optimal conditions of the above-mentioned precipitation stage, the yield and iodine adsorption values ​​were taken as the indicators, and the activation time, activation temperature and heating rate were selected. Three levels were selected and the L9(34) orthogonal test was carried out to investigate. The primary and secondary impact of each factor. Table 2 shows that the yield is the object of investigation. It can be seen from the range R that the primary and secondary order of each influencing factor is B>C>A, ie activation temperature>heating rate>activation time. It shows that the activation temperature has the greatest influence on the yield. The temperature is too high. A large amount of C is removed in the form of CO2 and H2O, which makes the yield decrease. If the temperature is too low, the activation is incomplete, the lost small molecules are small, and C is retained. Down, so the rate will be very high. In terms of yield, the higher the yield, the better. It can be seen from Table 2 that the optimum process conditions are B1C2A, ie activation temperature 400 °C, heating rate 10 °C/min, activation time 50 min; verification test according to B1C2A1, the result is The rate was 64.346% and the iodine value was 726.58 mg/g.
Taking the iodine adsorption value as the object of investigation, it can be seen from the range R' that the primary and secondary order of each influencing factor is B>A>C, ie activation temperature>activation time>heating rate. It indicates that the activation temperature has the greatest influence on the iodine adsorption value. The reason may be that temperature is the main factor of pore formation during activation. As the temperature increases, the reaction rate of carbonization and activation increases, and a large number of micropores are formed. However, if the temperature is too high, the pores become larger, and the iodine adsorption capacity decreases. . In terms of iodine adsorption value, the higher the iodine adsorption value, the better the adsorption performance of activated carbon. It can be seen from Table 2 that the optimum process condition is B3A2C1, that is, the activation temperature is 600 ° C, the activation time is 70 min, and the heating rate is 20 ° C/min. The verification test was carried out according to B3A2C1, and the yield was 66.32%, and the iodine adsorption value was 744.38 mg/g. Considering the above situation, the optimal preparation conditions are: activation temperature 600 ° C, activation time 70 min, heating rate 10 ° C / min. Two parallel experiments were carried out under the above optimum conditions to obtain activated carbon having a yield of 48.84% and an iodine value of 807.8 mg/g.
Table 2 Orthogonal test results and analysis in the activation phase
2.3 Determination of wood activated carbon related indicators
The optimum process conditions of the aging and activation stages were selected, namely, the aging time of 12 h, the aging temperature of 120 ° C, the impregnation ratio of 1:3, the zinc chloride solution concentration of 40%, the activation temperature of 600 ° C, the activation time of 70 min, and the heating rate of 10 ° C / Min, under the conditions of the preparation of activated carbon, and the relevant indicators were measured, the yield was 46.39%, ash 5.29%, iodine value 839.68mg / g, methylene blue value 5.5ml / 0.1g and A method caramel decolorization rate of 120%. The test results show that it is feasible to prepare activated carbon from acid lignin as raw material and zinc chloride as activator.
3 Conclusion
(1) The optimal test conditions for the stage of aging are: 12 h for aging, 120 °C for aging, 1:3 for immersion, and 40% for zinc chloride. The temperature of the aging is the most important factor in this stage. The following are the time of aging, the ratio of impregnation, and the concentration of zinc chloride.
(2) The optimal test conditions for the activation phase are: activation temperature 600 ° C, activation time 70 min, and heating rate 10 ° C / min. The activation temperature has the greatest influence on the test index, followed by the activation time and the heating rate.
(3) Based on the above optimal test conditions, the relevant indicators of woody activated carbon were: yield of 46.39%, iodine adsorption value of 839.68 mg/g, methylene blue adsorption value of 5.5 ml/0.1 g, and decolorization rate of caramel of method A of 120 %, moisture 4.81%, ash 5.29%.
(4) Preparation of cotton pulp lignin activated carbon by ZnCl2 activation method is simple, saves resources, reduces environmental pollution, and has good adsorption effect on liquid phase impurities, and has broad application prospects.
Modern Soap Dispenser,Soap Dispenser For Vila,Soap Dispenser Pump For Sink,Kitchen Sink Soap Dispenser
Kaiping Jenor Sanitary Ware Co., Ltd , https://www.jmjenorsanitary.com