Biomass is the only renewable organic carbon resource and is an ideal alternative to oil-producing fuels and chemicals. Therefore, the development of new routes and new methods for the conversion of biomass to fuels and chemicals is an important goal for the future development of a sustainable energy system.
However, the conversion of hydrodeoxygenated oxygen-enriched biomass feedstocks into petroleum-based products requires a large amount of precious metal catalysts such as platinum, palladium, rhodium, etc., which has become an important bottleneck for large-scale biorefinery applications. Therefore, the significant reduction of the use of precious metal catalysts in the conversion of biomass feedstocks and the ultimate use of the abundant "cheap" metal elements on the planet to completely replace precious metals have become major challenges in this field.
The existing research results show that non-noble metals have excellent hydrogenation properties, however, due to the easy agglomeration and loss of non-noble metals during the liquid phase catalytic process, the catalyst quickly loses its catalytic reactivity.
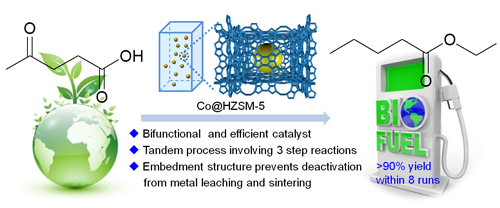
State Key Laboratory of Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences Li Fuwei The “Hundred Talents Program†project team has made new advances in the substitution of precious metals. They coated non-precious metal nano-cobalt into acid HZSM-5 zeolite crystallites for the conversion of the biomass platform compound levulinic acid to valerate (valerates are very suitable as additives for gasoline or diesel oil, have already passed Shell The company's 250,000 km road test is hailed as a new generation of cellulose-based transportation fuels.
The reaction process is carried out under liquid phase conditions and the loss of non-noble metals loaded by the impregnation process is severe. However, encapsulating the nanocobalt into the molecular sieve grains can inhibit the agglomeration and loss of metal due to the confinement effect, while the pore structure of the molecular sieve provides a molecular transport channel, so that the metal is in effective contact with the reactant molecules. The activity and stability of the catalyst were tested in a tank reactor and a fixed bed continuous reactor, respectively. The levulinic acid was completely converted, the product selectivity was over 90%, and the catalyst could be operated stably. This study provides an effective approach for the replacement of precious metals in petroleum refining and biorefinery. The results were published in ACS Catalysis (2014, 4 (11), 4136-4142) and have applied for Chinese invention patents.
The team has been working on the conversion of biomass and its derivatives into fuels and chemicals in recent years. Through multi-component direct carbonation, Ru-Ni bimetallic based ordered mesoporous carbon catalysts with uniform dispersion, controllable composition and in-situ production have been successfully prepared. The catalyst was used for the preparation of γ-valerolactone by hydrogenation of levulinic acid. The single reaction TON value was about 59,000. The catalyst showed good stability. After 15 times of repeated use, no significant decrease in activity was observed. It is worth mentioning that the reaction can be carried out in water, and only the water by-products are generated during the entire reaction process, perfectly embodying the concept of green chemistry. The high activity, high stability of the catalyst, and the environmental friendliness of the reaction system have laid a good foundation for further amplification of the reaction system. The related work was published in ACS Catalysis (2014, 4 (5), 1419-1425) and applied for one Chinese invention patent.

In addition, the team also developed a "metal-solid acid" bifunctional catalyst for the direct catalytic conversion of cellulose "one-pot" to prepare high value-added polyester monomer isosorbide, a water-resistant, strongly acidic mesoporous phosphate that has been developed. The ruthenium supported ruthenium catalysts can maintain strong acidity and structural stability in a hydrothermal environment, and can efficiently hydrolyze microcrystalline cellulose and catalyze subsequent hydrogenation and dehydration reactions without the use of liquid acids. The yield of isosorbide is up to 52%, which is one of the best results reported so far. The catalyst can be used in many stable cycles. This work was selected by ChemSusChem as a cover (2013, 6(11), 2190-2197) and applied for two Chinese invention patents.

The above work has been funded by the Chinese Academy of Sciences “Hundred Talents Programâ€, the National Natural Science Foundation of China (21002106, 21133011, and 21373246), Lanzhou Institute of Chemical Industry “1-3â€, and the Jiangsu Provincial Natural Science Foundation (BK20130354).
Low Temperature Welding Vacuum Glass
The key of vacuum glass is the glass welding technology. Therefore, the low temperature welding of Safety Vacuum Glass has always been the research focus at home and abroad, and it is the inevitable requirement of the development of building glass industry. The vacuum glass from ICESUN adopts low-temperature welding technology and professional welding equipment. We not only master the core technology and break the gap in this field in China, but also greatly reduce the cost through the cooperation of the industrial chain and lay the foundation for industrialization. ICESUN tempered vacuum glass is separated by a 0.2mm high support, and the edges of the two layers of glass plates are sealed with low-temperature glass, and then the air is discharged. If any need for Vacuum Glass For Passive House, Vacuum Glass For Sun Room , Vacuum Glass For Greenhouse, Vacuum Glass For Cars or other Environmental Vacuum Glass products, please contact ICESUN Vacuum Glass LTD. at any time you need.

Low Temperature Welding Vacuum Glass,Low Temperature Welding Glass,Patented Low Temperature Welding Glass,Low-Temperature Welding Sealed Vacuum Glass
ICESUN VACUUM GLASS LTD. , https://www.icesunvacuumglass.com