- Iron-carbon alloys are the most widely used metal materials for humans. Different compositions of iron-carbon alloys have different structures and properties. In the research and use of steel materials, the development of its thermal processing and heat treatment processes, and the analysis of process waste, it is necessary to apply the iron-carbon phase diagram, showing the importance of the iron-carbon phase diagram, the following small series take everyone from iron-carbon The typical structure of the alloy, the phase diagram analysis and the equilibrium crystallization process take a look at the iron-carbon phase diagrams we have pursued in those years.
 Iron-carbon alloy typical organization
Iron-carbon alloy typical organization
Ferrite / (α-Fe) / (F)
Tissue: solid solution of carbon in α-Fe, body-centered cubic lattice;
Characteristics: carbon has very little solubility in α-Fe, only 0.0008% at room temperature, and reaches a maximum solubility of 0.0218% at 727 °C;
Performance: The mechanical properties of ferrite are characterized by good plasticity and toughness, while low strength and hardness.
The microstructure of the ferrite is a polygonal grain and its performance is similar to that of pure iron.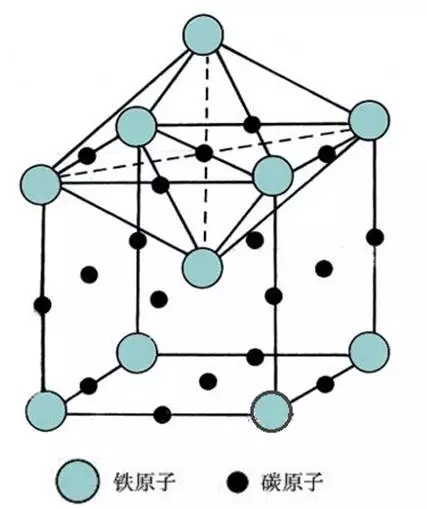 Ferrite crystal structure
Ferrite crystal structure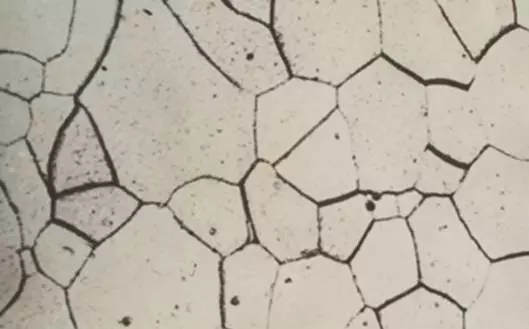 Ferrite metallographic diagram
Ferrite metallographic diagram
Austenite / (γ-Fe) / (A)
Tissue: carbon is dissolved in the interstitial solid solution in γ-Fe, face-centered cubic lattice;
Characteristics: The solubility of carbon in γ-Fe is larger than that in α-Fe, 0.77% at 727 °C, and the highest at 1148 °C, up to 2.11%;
Performance: Has a certain strength and hardness, plasticity and toughness.
The austenite structure is irregular polyhedral grains, and the grain boundary is processed in the austenite region compared with the straight steel.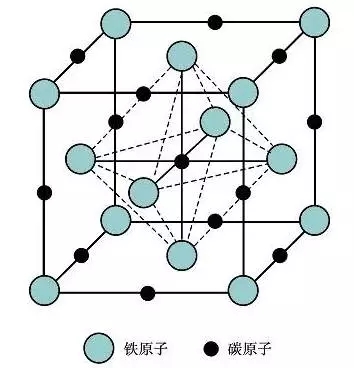 Austenitic crystal structure
Austenitic crystal structure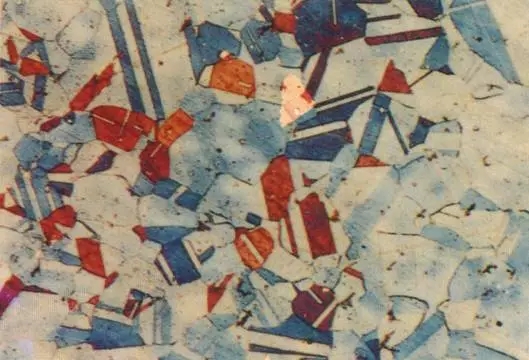 Austenitic metallographic diagram
Austenitic metallographic diagram
Cementite / (Fe3C) / (C)
Tissue: a compound of iron and carbon (Fe3C);
Characteristics: a gap compound with a complex lattice structure. The carbon content is 6.67%. Fe3C is a metastable phase, which will decompose under certain conditions;
Performance: High hardness, wear resistance, but brittleness, almost zero plasticity.
The cementite is a strengthening phase in the steel, and the cementite has a shape such as a strip, a mesh, a sheet, or a pellet depending on the production conditions.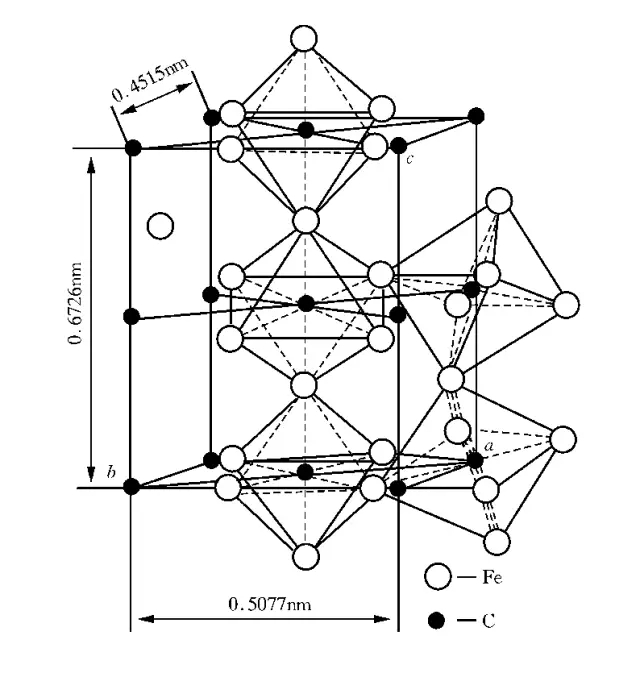 Cementite gold crystal structure
Cementite gold crystal structure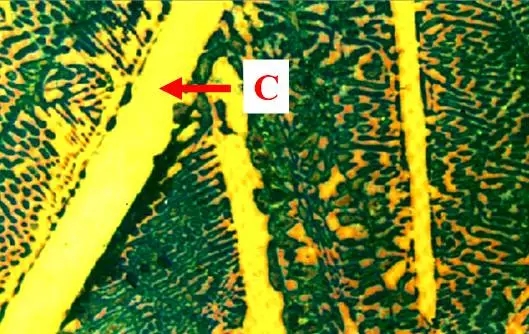 Carburite metallographic diagram
Carburite metallographic diagram
Pearlite (Pearlite) / (P)
Tissue: a mechanical mixture consisting of ferrite + cementite;
Characteristics: Pearlite is an isothermal transformation product of supercooled austenite, showing pearly luster. According to the transition temperature, pearlite is divided into: pearlite (P), sorbite (S) and troostite (T). There is no difference in the nature, the transition temperature is gradually reduced, the size P>S>T,;
Performance: The mechanical properties are between ferrite and cementite, with high strength, moderate hardness, good plasticity and toughness;
The microstructure is a sheet-like structure in which ferrite sheets and cementite sheets are alternately arranged, and spherical pearlite (also called granular pearlite) can also be obtained after spheroidizing annealing of high carbon steel. Flaky pearlite metallographic diagram
Flaky pearlite metallographic diagram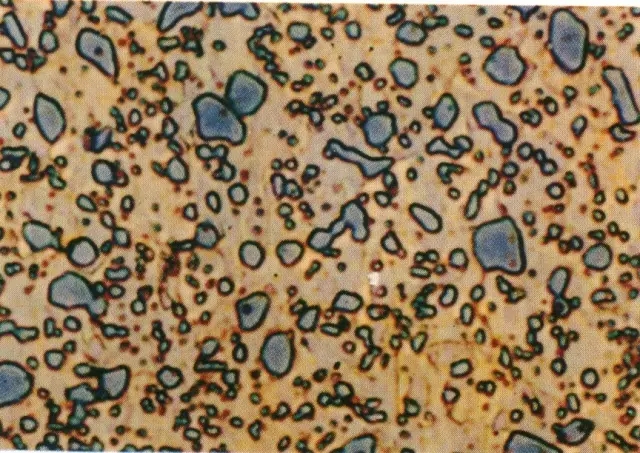 Granular pearlite metallographic diagram
Granular pearlite metallographic diagram
Ledeburite / (Ld / Ld')
Tissue: Leysite is a mechanical mixture of austenite + cementite, below 727 ° C, is a pearlite + cementite mechanical mixture;
Characteristics: When the carbon content of the cast iron alloy solution is above 2.11%, the eutectic lysite is solidified slowly to 1147 °C; the Leysite between 1148 °C and 727 °C is called high temperature lysite (Ld); 727 The Leysite below °C is called metamorphic Leysite or low temperature Leysite (Ld').
Properties: The mechanical properties of the Leysite are similar to those of cementite, the hardness is very high, and the plasticity is extremely poor, almost zero.
The metallographic structure is honeycomb-shaped as a whole, and the austenite is distributed on the matrix of the cementite.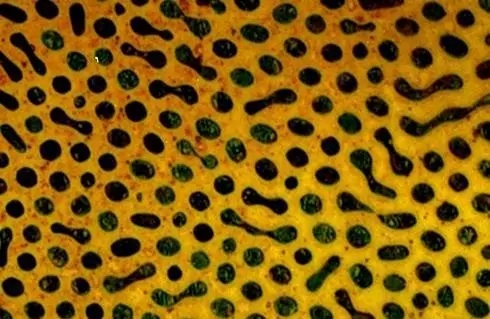 Leysite metallographic diagram
Leysite metallographic diagram
Bainite/(B)
Tissue: Bainite is a mechanical mixture of ferrite + cementite, a structure between pearlite and martensite;
Upper bainite: formed at 550~450 °C, the matrix is ​​ferrite, and the strip carbide precipitates on the edge of the ferrite sheet and is feathery;
Lower bainite: formed at 300 ° C, in the form of fine needles, acicular ferrite is covered with small pieces of carbide, the flaky carbides on the long axis of ferrite is roughly 55 ~ 60 degrees;
Granular bainite: The shape is equivalent to a polygonal ferrite, and the ferrite matrix is ​​covered with granular carbides (the small island structure is originally carbon-rich austenite, decomposed into ferrite and carbide when cooled, or transformed Martensite or still carbon-rich austenite particles)
Properties: The strength of the upper bainite is smaller than that of the fine pearlite formed at the same temperature, and the brittleness is large; in the low temperature range, the lower bainite obtained by the bainite transformation has very good comprehensive mechanical properties. Upper bainite metallography
Upper bainite metallography Lower bainite metallography
Lower bainite metallography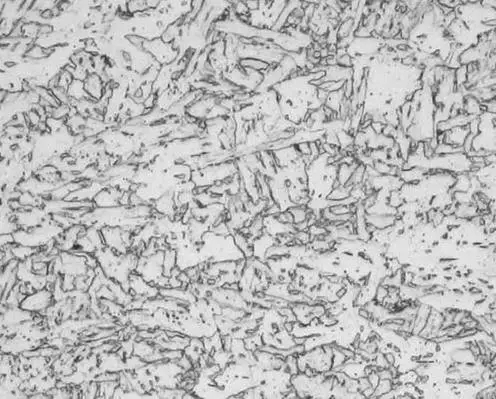 Granular bainite metallographic diagram
Granular bainite metallographic diagram
Weid organization (WidmannstattenStructure)
Tissue: a crystal form of a triangular, square or cruciform distribution in which a second phase precipitates along a certain crystal plane of the parent phase when the solid solution is decomposed;
Ferrite Weiss microstructure: In the hypoeutectoid steel, when the austenite passes through the Ar3 to Ar1 temperature zone at a rapid cooling rate, the ferrite sheets are inserted into the austenite grains, and these are distributed in the prior austenite grains. The flaky pro-eutectoid ferrite inside the granule is called ferritic Weiss tissue;
Cementite Wei's structure: In the hypereutectoid steel, when the austenite grain size and cooling conditions are suitable, the cementite appears in the form of needles or flat sheets and strips inside the austenite grains to form an infiltration. Carbon body Weishi tissue.
Performance: The coarse Wei's structure reduces the plasticity and toughness of the steel and increases the brittleness. Wei's organization metallographic map
Wei's organization metallographic map
Martensite / (M)
Tissue: The supersaturated solid solution of carbon in α-Fe is called martensite, body-centered square structure;
Characteristics: Martensite is a product of rapid cooling of supercooled austenite, a shearing mode between Ms and Mf points, which is divided into lath martensite (low carbon) and acicular martensite;
Properties: Martensite has high strength and hardness, but its plasticity is very poor, almost zero, and cannot withstand impact loads.
Lath martensite: Also known as low carbon martensite, formed in low, medium carbon steel and stainless steel, consisting of a plurality of bundles of slats arranged in parallel with each other. The shape of the space is flat, and an austenite grain can be transformed into several lath bundles (usually 3 to 5);
Needle martensite: also known as flaky martensite or high carbon martensite, flake martensite is common in high and medium carbon steels and high Ni Fe-Ni alloys; when the largest size martensite tablets When the optical microscope is indistinguishable, it is called cryptocrystalline martensite. The martensite obtained by normal quenching in production is generally cryptocrystalline martensite. Lath martensite metallographic diagram
Lath martensite metallographic diagram Acicular martensitic metallographic map
Acicular martensitic metallographic map
The martensite formed after quenching can also form three special metallographic structures after tempering:
Tempered martensite: refers to the formation of flake martensite (crystal structure is tetragonal) in the first stage of tempering, in which carbon is desolvated in the form of transition carbides. The solid solution matrix (the crystal structure has become the body-centered cubic) is dispersed in a multi-phase structure of extremely fine transition carbide flakes (the interface with the matrix is ​​a coherent interface); this structure is even under a metallographic (optical) microscope Zooming to the maximum magnification also does not distinguish its internal structure, only seeing that the whole is a black needle (the shape of the black needle is basically the same as the white needle formed by the martensite (also known as "α martensite") formed during quenching). ), this black needle is called "tempered martensite." Tempered martensite metallographic diagram
Tempered martensite metallographic diagram
Tempered sorbite: a product of quenched martensite after high temperature tempering. It is characterized in that the saoliteite matrix is ​​covered with fine granular carbides, which can be clearly distinguished under the light microscope. This type of tissue, also known as tempering tissue, has a good combination of strength and toughness. The finer the fine-grained carbide on the ferrite is, the hardness and strength are slightly higher, and the toughness is slightly worse. On the contrary, the hardness and strength are lower, and the toughness is higher. Tempered sorbite metallographic diagram
Tempered sorbite metallographic diagram
Tempered troostite: a product of quenching martensite by moderate temperature tempering, characterized in that the martensite needle-like morphology will gradually disappear, but it is still faintly visible (including chromium alloy steel, the recrystallization temperature of its alloy ferrite) It is higher, so it still maintains the needle-like shape. The precipitated carbide is small and difficult to distinguish clearly under the light microscope. Only the carbide particles can be seen under the electron microscope, which is highly susceptible to erosion and darkens the tissue. If the tempering temperature is upper limit or the retention time is slightly longer, the needles are white; at this time, the carbides are segregated at the edge of the needles, and the hardness of the steel is slightly lower and the strength is lowered.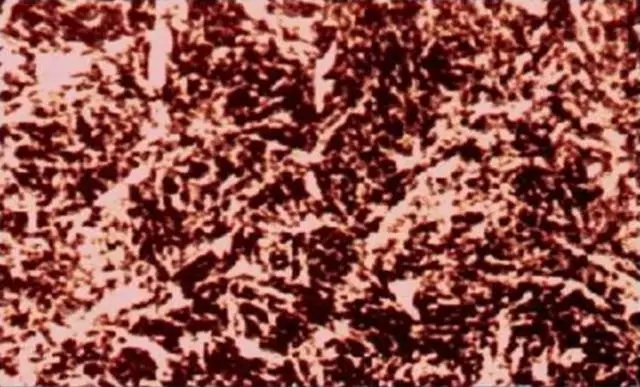 Tempered cucurbit metallographic diagramAnalysis of iron-carbon phase diagram
Tempered cucurbit metallographic diagramAnalysis of iron-carbon phase diagramIron-carbon phase diagram
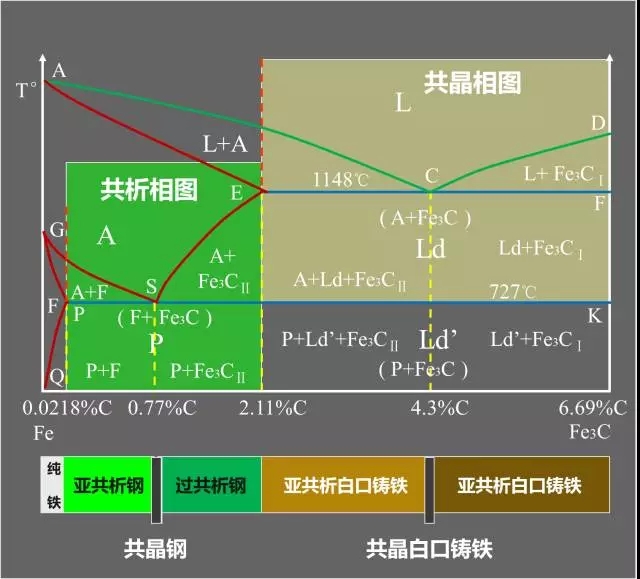
Classification of Fe-C alloys based on Fe-Fe3C phase diagram: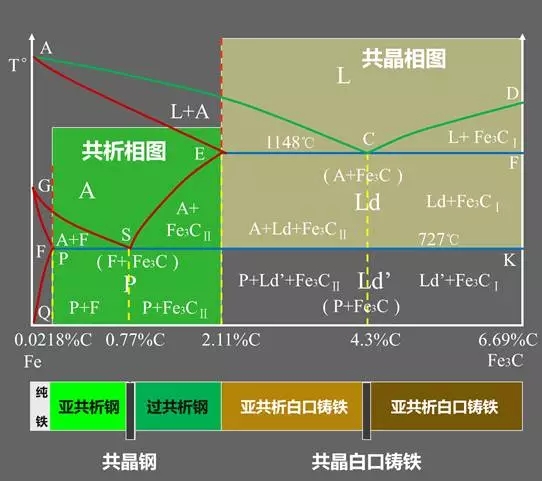
Industrial Pure Iron (carbon containing <0.0218%);
Hypo-Eutectoid Steel (carbon content <0.77%);
Eutectoid Steel (with a carbon content of 0.77%);
Hyper-Eutectoid Steel (carbon content >0.77%);
Hypo-Eutectic White Cast Iron (carbon content <4.3%);
Eutectic White Cast Iron (4.3% carbon);
Hyper-Eutectic White Cast Iron (carbon >4.3%).
Three major reactions:
Peritectic Reactions
The first solid solution is formed by reacting with the surrounding residual liquid to form a second solid solution, and the original primary crystal is wrapped;
The peritectic reaction formula: LB + δ → AJ.
Eutectic Reactions
It is the simultaneous precipitation of two different solids from one liquid;
Eutectic reaction formula: LC → AE + Fe3C.
Eutectoid Reactions (EutectoidReactions)
It is the simultaneous precipitation of two different solids from one solid;
Eutectoid reaction formula: AS → FP + Fe3C.Feature point summary table
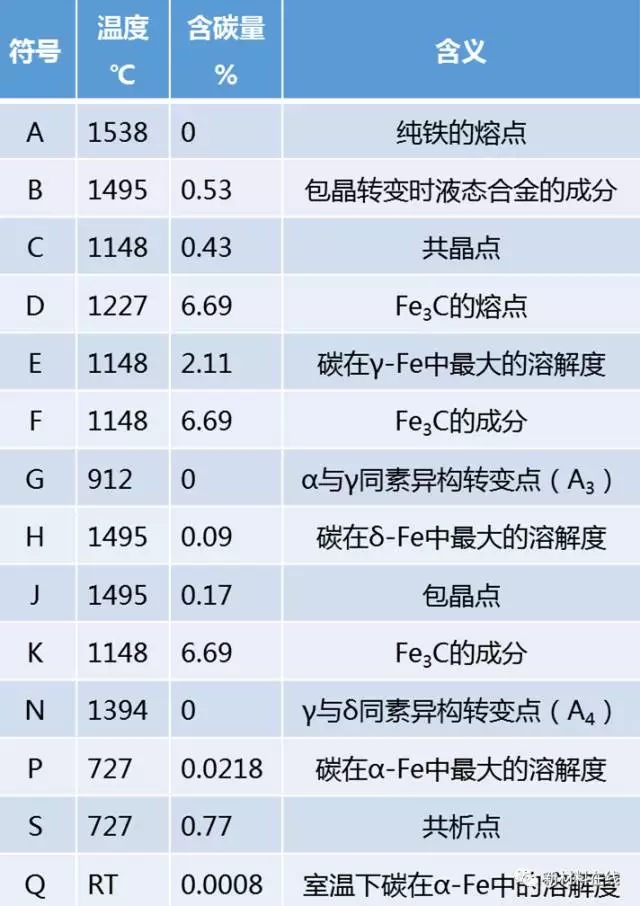
Feature line summary table Equilibrium crystallization process of iron-carbon alloy
Equilibrium crystallization process of iron-carbon alloy
Industrial pure iron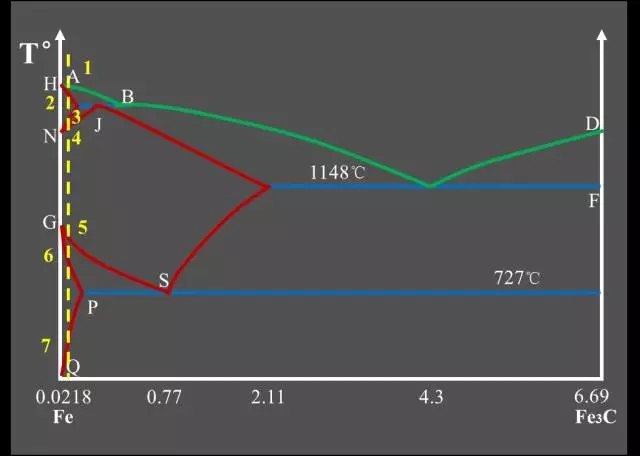
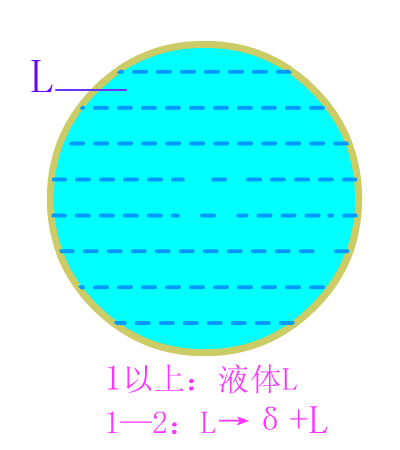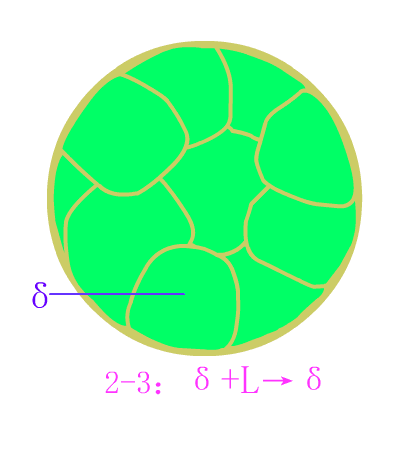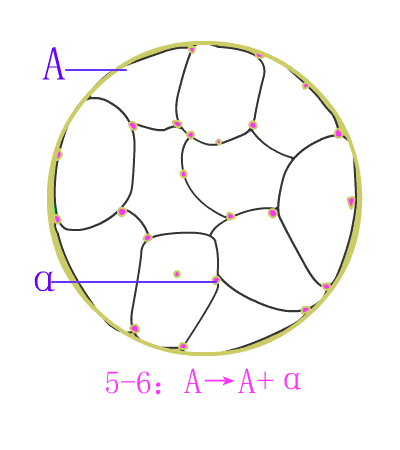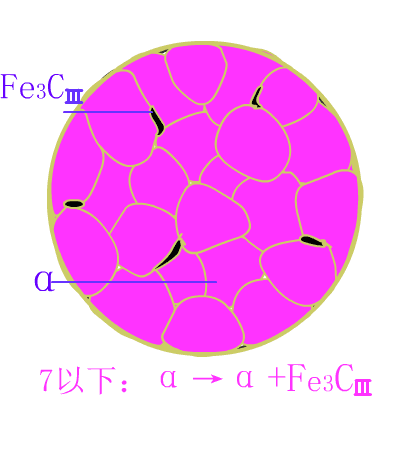 Industrial pure iron equilibrium crystallization diagram
Industrial pure iron equilibrium crystallization diagram
The industrial pure iron is organized at room temperature: ferrite (F) + tertiary cementite (Fe3CIII) . Typical metallographic diagram of industrial pure iron
Typical metallographic diagram of industrial pure iron
Eutectic steel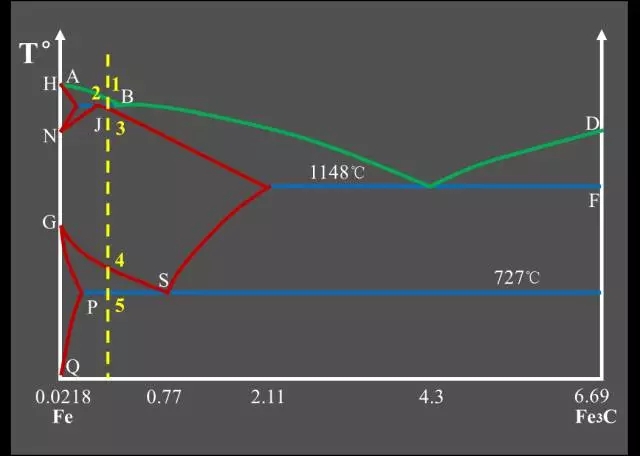
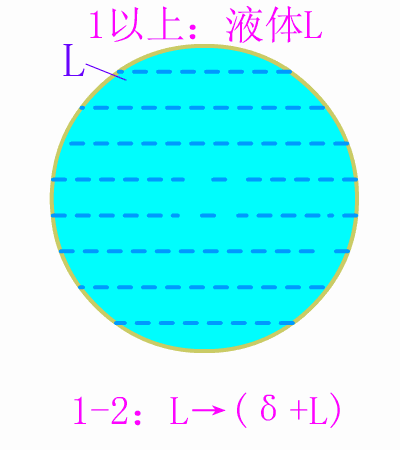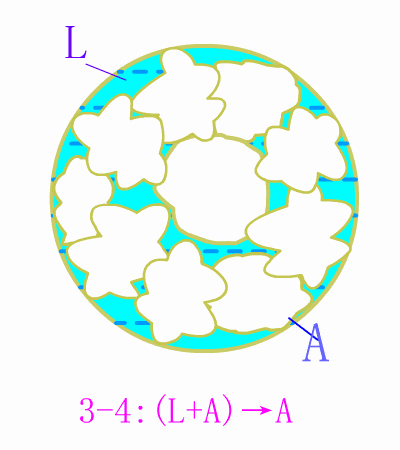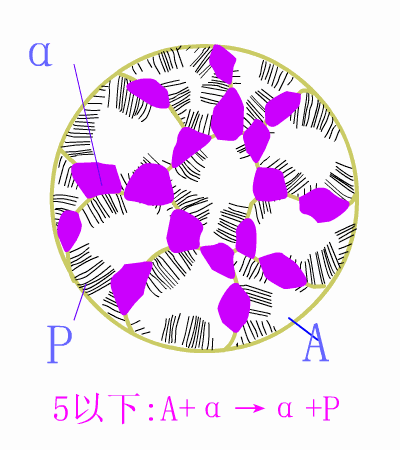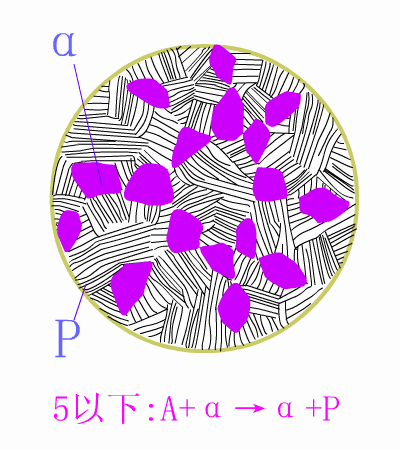 Schematic diagram of equilibrium precipitation of hypoeutectic steel
Schematic diagram of equilibrium precipitation of hypoeutectic steel Typical metallographic diagram of hypoeutectic steel
Typical metallographic diagram of hypoeutectic steel
The basic reaction of the eutectic steel crystallization process is: homogenization reaction + peritectic reaction + solid solution transformation reaction + eutectoid reaction .
Room temperature equilibrium structure of hypoeutectoid steel: ferrite (F) + pearlite (P) is precipitated first, and the amount of P increases with the increase of carbon content .
Eutectoid steel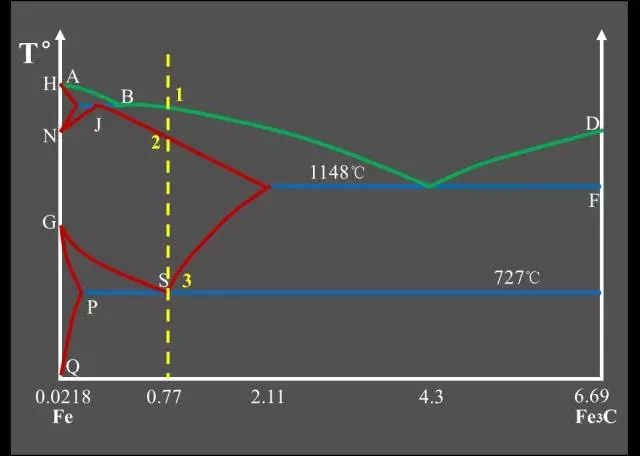
 Eutectic steel equilibrium crystallization diagram
Eutectic steel equilibrium crystallization diagram
The basic reaction of the crystallization process of the eutectoid steel is: homogenization reaction + eutectoid reaction.
The room temperature structure of the eutectoid steel is: 100% pearlite (P), the relative weight ratio of ferrite to cementite phase is 8:1 . Typical metallographic diagram of eutectoid steel
Typical metallographic diagram of eutectoid steel
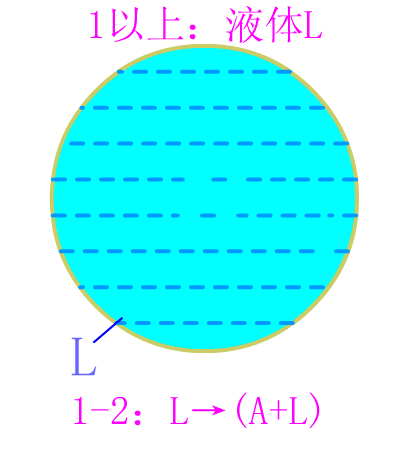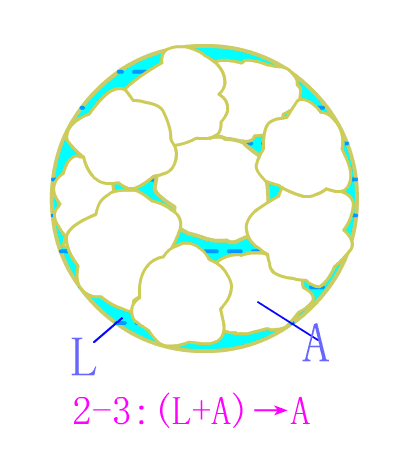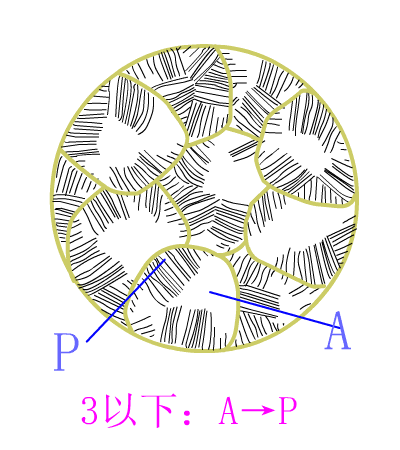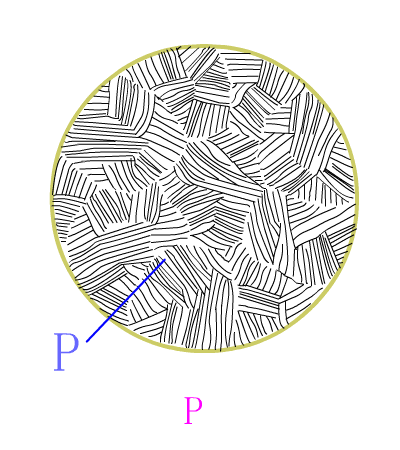
Hypereutectoid steel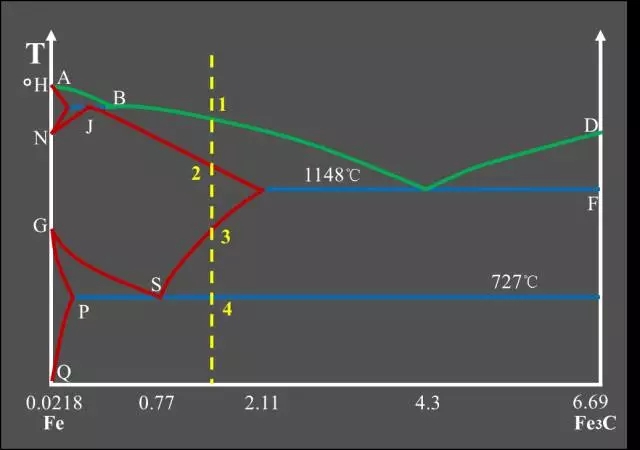
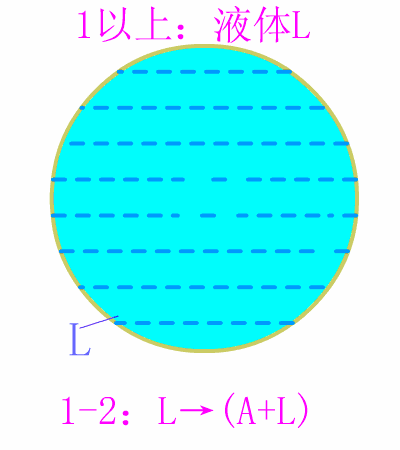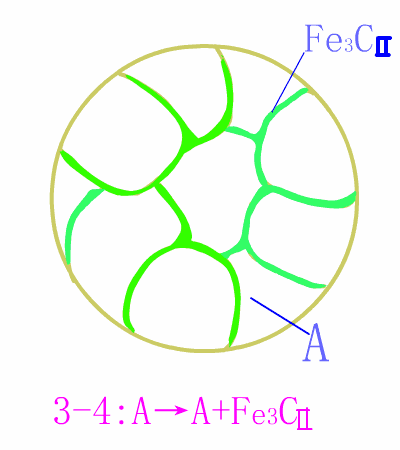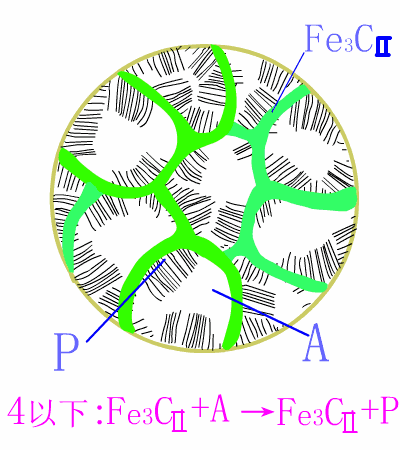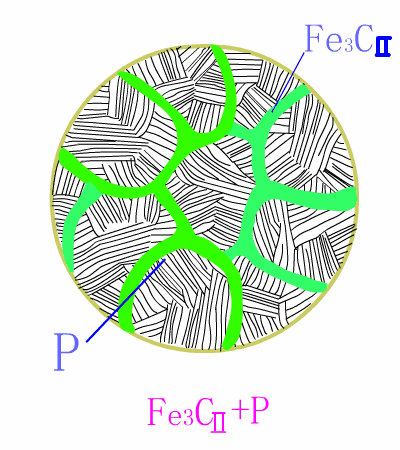 Schematic diagram of equilibrium eutectoid steel equilibrium crystallization
Schematic diagram of equilibrium eutectoid steel equilibrium crystallization
The basic reaction of the eutectic steel crystallization process is: homogenization reaction + secondary precipitation reaction + eutectoid reaction .The room temperature structure of the eutectoid steel is: pearlite (P) + secondary cementite (Fe3CII) , and Fe3CII precipitates along the austenite grain boundary, which makes the overall brittleness of the material increase.

Typical metallographic diagram of hypereutectoid steel
Hypoeutectic white cast iron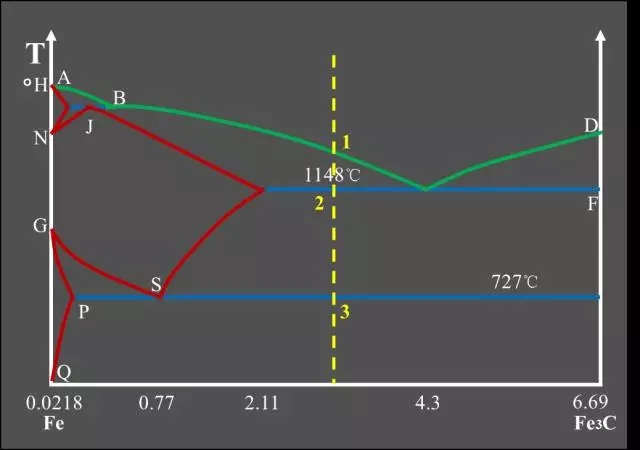
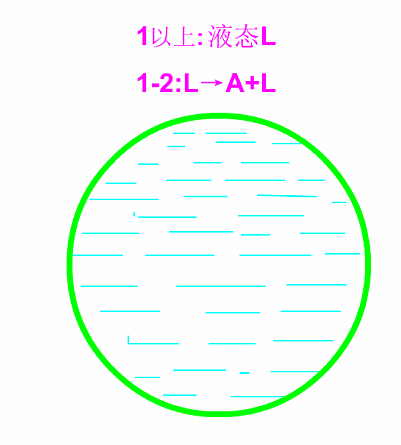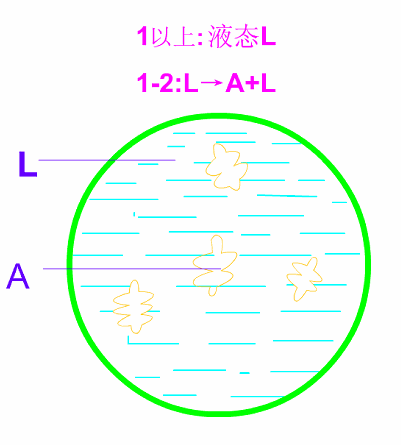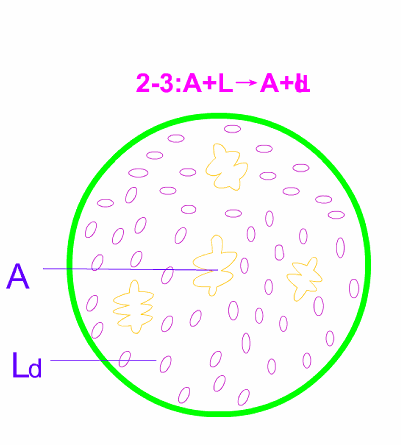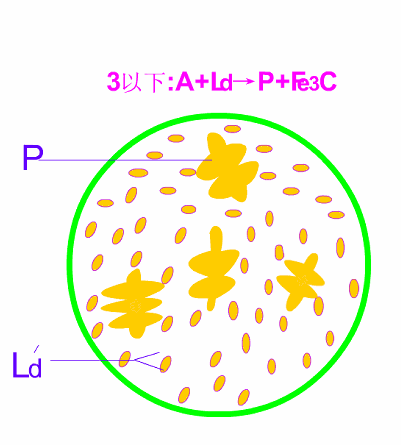 Schematic diagram of equilibrium crystallization of hypotectic cast iron
Schematic diagram of equilibrium crystallization of hypotectic cast iron
The basic reaction of the eutectic white cast iron crystallization process is: homogenization reaction + eutectic reaction + secondary precipitation reaction + eutectoid reaction .
The room temperature structure of hypoeutectic white cast iron is: pearlite (P) + secondary cementite (Fe3CII) + low temperature lysite (Ld') .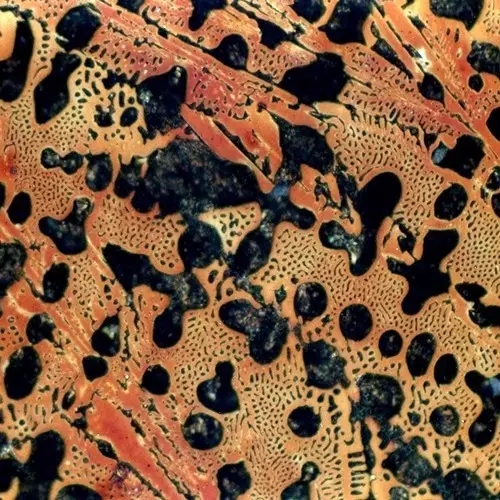 Typical metallographic diagram of hypoeutectic cast iron
Typical metallographic diagram of hypoeutectic cast iron
Eutectic white cast iron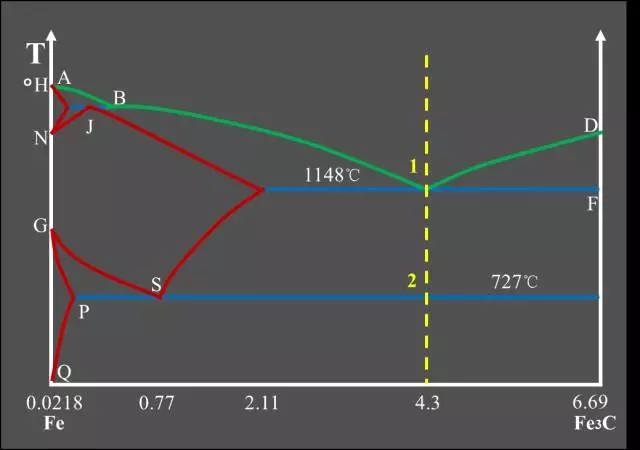 Schematic diagram of eutectic cast iron equilibrium
Schematic diagram of eutectic cast iron equilibrium
The basic reaction of the crystallization process of eutectic white cast iron is: eutectic reaction + secondary precipitation reaction + eutectoid reaction .
The room temperature structure of eutectic white cast iron is: low temperature lysite (Ld') .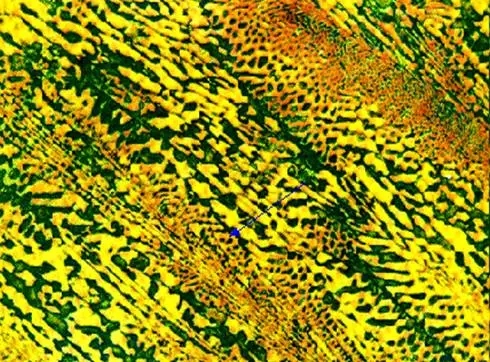 Typical metallographic diagram of eutectic cast iron
Typical metallographic diagram of eutectic cast iron
Hypereutectic white cast iron
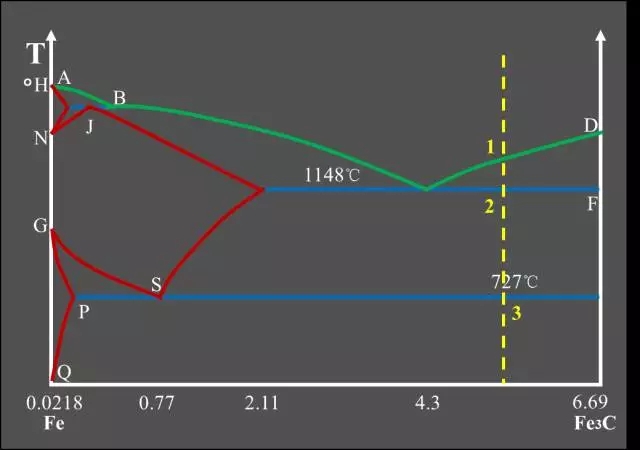
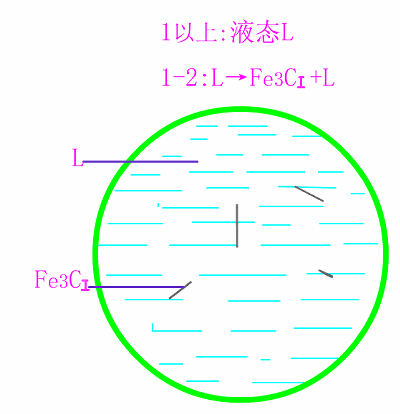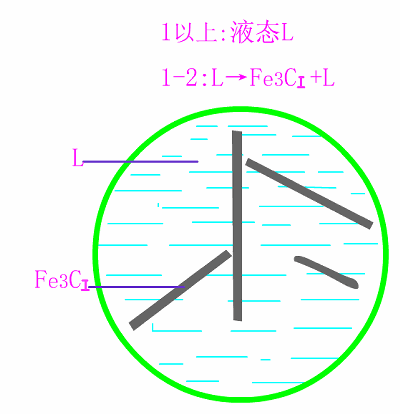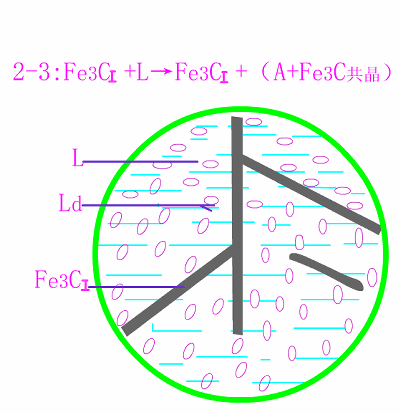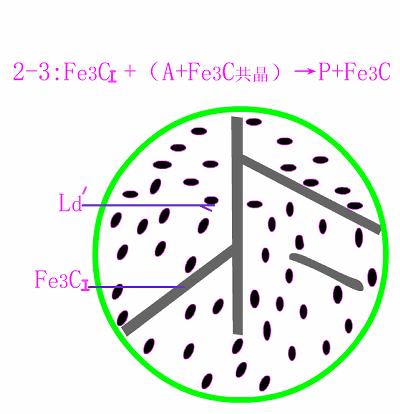 Schematic diagram of equilibrium crystallization of hypereutectic cast
Schematic diagram of equilibrium crystallization of hypereutectic cast
The basic reaction of the crystallization process of hypereutectic white cast iron is: homogenization reaction + eutectic reaction + secondary precipitation reaction + eutectoid reaction .
The room temperature structure of hypereutectic white cast iron is: primary cementite (Fe3C) + low temperature lysite (Ld') . Typical metallographic diagram of hypereutectic white cast iron
Typical metallographic diagram of hypereutectic white cast iron
The main advantage of bioglass ceramic tube is that CaO and P2O5 can be introduced into the glass. Hydroxyapatite crystals can be precipitated by heat treatment. It has excellent biocompatibility and bioactivating properties, and other components in the composition can precipitate other components. Types of crystals ensure the chemical stability and machinability of materials, and are more promising than metals, alumina and Other Materials. Many clinical trials have been conducted to date, some for six years, and have yielded promising results.
Machinable Glass Ceramic Tube Dia,Machinable Glass Ceramic Macor Tube,Machinable Glass Ceramic Tube Bushing
Dongguan Haikun New Material Co., Ltd. , https://www.hkceram.com